Oncoscience
Activity of pazopanib in EWSR1-NFATC2 translocation-associated bone sarcoma
Mohamed A. Gouda1, Maria A. Zarzour2, Ara A. Vaporciyan3, Kalevi Kairemo4, Hubert H. Chuang4 and Vivek Subbiah1,5
1 Department of Investigational Cancer Therapeutics, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA
2 Department of Sarcoma Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA
3 Department of Thoracic and Cardiovascular Surgery, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA
4 Department of Nuclear Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA
5 Sarah Cannon Research Institute, Nashville, TN 37203, USA
Correspondence to: Vivek Subbiah, email: [email protected]
Keywords: pazopanib; precision oncology; sarcoma
Received: June 08, 2023
Accepted: August 21, 2023
Published: September 20, 2023
Copyright: © 2023 Gouda et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
Pazopanib is a multi-kinase inhibitor that is currently approved for treatment of advanced renal cell carcinoma and chemotherapy-refractory soft tissue sarcoma. In this case report, we discuss the case of a patient with a EWSR1-NFATC2 fusion positive bone sarcoma who had exceptional tumor control through using pazopanib and surgery for an overall duration exceeding 5 years. We also review the literature on EWSR1-NFATC2 translocation-associated sarcomas and use of pazopanib in bone sarcomas.
INTRODUCTION
Bone sarcomas are a rare but aggressive group of cancers that strike adolescents and young adults in the prime of their lives [1–4]. There is a wide spectrum of histological diagnoses although osteosarcoma and Ewing sarcoma are the most common sub-types [5]. Beyond chemotherapeutic agents, unprecedented advances in immunotherapy and genomically targeted therapy that have conferred clinical benefit in many epithelial cancers have had minimal impact in the outlook of metastatic/relapsed bone sarcomas [6, 7]. Therefore, exploring other potential treatment options that can be used in bone sarcoma especially in the setting of molecularly driven therapeutics is needed.
Pazopanib is a multi-kinase inhibitor that works by targeting vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), and c-KIT; hence, inducing an antiangiogenic effect that leads to inhibition of tumor growth and apoptosis [8]. Pazopanib is currently approved by the United States Food and Drug Administration (FDA) for treatment of advanced renal cell carcinoma and advanced chemotherapy-refractory soft tissue sarcoma. However, it has been used off-label in many bone sarcomas and a few of them have been reported to derive clinical benefit. Molecular profiling and biomarkers may aid in understanding not only the diagnosis but also the underlying the response and/or resistance mechanisms [9].
In this case study, we report a patient with a EWSR1-NFATC2 fusion positive bone sarcoma who had exceptional tumor control through using pazopanib and surgery for an overall duration exceeding 5 years. We also review the literature on EWSR1-NFATC2 translocation-associated sarcomas and use of pazopanib in bone sarcomas.
CASE PRESENTATION
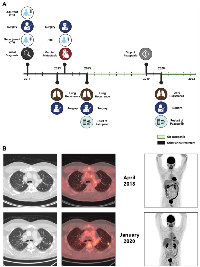
A previously healthy male in his 30s initially presented with left leg mass. A biopsy was suggestive of high-grade bone sarcoma with small cell features. Patient received preoperative standard of care chemotherapy with vincristine, doxorubicin, cyclophosphamide, ifosfamide, and etoposide but showed minor tumor response and 10 to 20% necrosis in pathological analysis of the below knee amputation surgical specimen. He then received adjuvant chemotherapy with high dose ifosfamide alternating with doxorubicin and cisplatin but unfortunately developed lung recurrence after 2 years. Patient underwent wedge resection of lung metastasis that was consistent with the initial diagnosis of metastatic small round cell carcinoma. A comprehensive genomic profiling showed EWSR1-NFATC2 fusion, mTOR E1799K mutation, and TOP1 amplification. During postoperative imaging, cardiac metastasis was identified. Treatment with temozolomide and irinotecan was initiated but minimal response was observed, and patient was referred for surgical resection of the cardiac metastasis. The metastasis was attached only to a tricuspid valve chordae tendineae and an R0 resection was performed. Given poor response to previous therapies, patient was not offered any further adjuvant treatment and elected to active surveillance. Sixteen months later, a lung metastasis recurred, and he underwent another surgical resection. Based on multiple reports on response to pazopanib in patients with bone sarcomas and patient’s wish to pursue adjuvant treatment, therapy with pazopanib 800 mg orally daily was initiated one month after surgery. Patient’s disease remained under control for 5 years and after extensive discussion with patient weighing pros and cons of treatment discontinuation, pazopanib was stopped. A few months after cessation of pazopanib, patient developed disease progression to the lung which was again treated with upper lung wedge resection. Pathology showed metastatic round cell tumor. Patient had an uneventful post-operative course. After surgery, patient restarted pazopanib and to date continue to have a disease-free status for 30 months based on PET/CT regular follow up imaging (Figure 1).
DISCUSSION
Pazopanib, a multi-kinase VEGF inhibitor, is currently FDA approved for advanced renal cell carcinoma and advanced soft tissue sarcoma; but limited evidence exists on its efficacy in bone sarcomas. Despite multiple preclinical studies supporting the biological rationale of using pazopanib [10–13], most clinical evidence comes from off label use reported in case reports and case series (Table 1). In addition, two phase 2 trials of pazopanib in bone sarcomas have shown promising results. Schulte et al. reported progression-free survival (PFS) at 12 weeks of 70% in an exploratory cohort of osteosarcomas receiving pazopanib and oral topotecan [median PFS = 4.5 months] [14]. Another phase 2 study (NCT01759303) suggested that 6 out of 12 patients with metastatic osteosarcoma had clinical benefit from pazopanib although the study was terminated early due to sponsor withdrawal [15].
Our patient showed a dramatic long-term response to pazopanib following multiple failed trials of chemotherapy that were followed with disease recurrence. Interruption of pazopanib led to interval disease progression which validates the contribution of pazopanib to long-term disease control. A review of literature shows the definitive benefit of pazopanib in anecdotal cases of bone sarcomas (Table 1). However, biomarker-based reporting has been only presented in few studies. Molecular profiling has shown a great potential in guiding treatment decisions including those in patients with bone tumors [30, 31]. A study by Egas-Bejar et al. [7] suggested that mutations in PI3K/PTEN/mTOR pathway are not uncommon in patients with osteosarcoma. Not only can genetic testing identify actionable alterations, but it can also help to molecularly characterize tumors’ behavior and establish prognostic subgrouping usable in clinical management [7]. In our case, the patient’s tumor harbored EWSR1-NFATC2 fusion, mTOR E1799K mutation, and TOP1 amplification. EWSR1 encodes for the EWS protein which plays a pivotal role in gene transcription. Alterations in EWSR1 gene, including gene rearrangements, have been commonly linked to cases with bone sarcomas via aberrant dysregulation of gene transcription leading to uncontrolled cellular growth and survival [32]. Most fusion partners that have been described belong to the ETS family of genes, including FLI1 and ERG genes, but more recently interest has grown in EWSR1-non-ETS fusions including EWSR1-NFATC2. In fact, newer evidence suggests that sarcomas with that EWSR1-NFATC2 have distinct tumor characteristics and should be considered as a separate disease entity from other bone sarcomas (Table 2). Translocation-associated small round cell sarcoma with EWSR1-NFATC2 fusion has been described to be resistant to conventional Ewing sarcoma chemotherapy [33]. A multiscale-omic analysis revealed upregulation of the mTOR pathway in those patients which presents another chance for therapeutic targetability in the era of precision oncology [34]. Interestingly, mTOR E1799K mutation was also observed in our patient. mTOR is an atypical protein kinase that is proposed to be linked to the PI3K signaling pathway dysregulation of which has been postulated as a potential mechanism for oncogenesis [35, 36]. In addition to EWSR1 and mTOR, TOP1 amplifications were also identified in our patient and previously hypothesized to associate with more aggressive tumors in patients with melanoma and responses to TOP1 inhibitors including topotecan and irinotecan [37].
With its multi-kinase activity, including actions on VEGF, PDGFR, FGFR, and KIT, pazopanib leads to a desirable inhibition of tumor growth which antagonizes the impact of such tumor-promoting alterations and possibly explain the derived clinical benefit in our patient. This benefit is probably derived from the action on VEGF which has been reported to be upregulated in patients with sarcoma [65, 66]. Since mTOR overactivation might have been the case in our patient either through the upregulation by EWSR1-NFATC2 fusion [34] or the co-occurring mTOR 1799K mutation, it is possible that such activation might have led to increased VEGF given the connection between both pathways [67]; which in turn yielded the tumor responsive to pazopanib. Moreover, there is at least some evidence that other EWS fusions, e.g., EWS-FLI, are directly associated with increase in VEGF and tumor-associated angiogenesis [65, 66]. Responses to pazopanib inhibition have been reported in non-bone sarcomas with EWS-ATF1 and EWSR1-CREB1 fusions [68, 69]. Interestingly, a cell line Hewga-CCS was established from a clear cell sarcoma harbored the type 2 EWS-ATF1 transcript. In the pre-clinical studies reported, pazopanib suppressed the growth of these cell lines both in vivo and in vitro. Intriguingly, A phospho-receptor tyrosine kinase array revealed phosphorylation of c-MET, but not of VEGFR in these models and ensuing experiments revealed that pazopanib exerted antitumor effects through the inhibition of HGF/c-MET signaling [69]. It is possible that other related fusions, including the EWSR1-NFATC2 fusion detected in our patient, may confer a shared inherent potential sensitivity to pazopanib via its association with VEGF and perhaps other pathways like HGF-c-MET.
In brief, this case, in accordance with previously reported evidence, provides proof of activity of pazopanib in EWSR1-NFATC2 positive sarcoma. The report shows that pazopanib when administered in an adjuvant capacity demonstrated its effectiveness in preventing or delaying the progression of additional metastasis. Nevertheless, due to the adjuvant nature of the treatment, it remains uncertain whether this approach would have resulted in tumor shrinkage. Further pre-clinical studies and clinical studies using pazopanib in EWSR1-NFATC2 sarcomas are warranted.
Author contributions
Authors have contributed to the conception and writing of this manuscript. All authors have approved the final manuscript draft.
ACKNOWLEDGMENTS AND FUNDING
Vivek Subbiah (VS) reports employment with UT MD Anderson Cancer Center at the time of submission and reports employment by Sarah Cannon Research Institute at the time of acceptance. VS is an Andrew Sabin Family Foundation Fellow at The University of Texas MD Anderson Cancer Center. VS acknowledges the support of The Jacquelyn A. Brady Fund. VS is supported by a US National Institutes of Health (NIH) grant (No. R01CA242845 and R01CA273168); MD Anderson Cancer Center Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention and Research Institute of Texas (No. RP1100584), the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (No. 1U01 CA180964), NCATS (Center for Clinical and Translational Sciences) Grant (No. UL1 TR000371), and the MD Anderson Cancer Center Support Grant (No. P30 CA016672). The included figure was created with https://www.biorender.com/.
CONFLICTS OF INTEREST
MAG, MAZ, KK, and HC have no conflicts of interest. AAV is on the advisory board for Astrazeneca. VS at the time of submission reports research funding from Novartis to conduct clinical trials; and other grant support for clinical trials from AbbVie, Agensys, Inc., Alfasigma, Altum, Amgen, Bayer, BERG Health, Blueprint Medicines Corporation, Boston Biomedical, Inc., Boston Pharmaceuticals, Celgene Corporation, D3 Bio, Inc., Dragonfly Therapeutics, Inc., Exelixis, Fujifilm, GlaxoSmithKline, Idera Pharmaceuticals, Inc., Incyte Corporation, Inhibrx, Loxo Oncology/Eli Lilly, MedImmune, MultiVir, Inc., NanoCarrier, Co., National Comprehensive Cancer Network, NCI-CTEP, Novartis, PharmaMar, Pfizer, Relay, Therapeutics, Roche/Genentech, Takeda, Turning Point Therapeutics, UT MD Anderson Cancer Center, and Vegenics Pty. Ltd.; as well as travel support from ASCO, ESMO, Helsinn Healthcare, Incyte Corporation, Novartis, and PharmaMar; and reports consultancy or advisory board participation for Helsinn Healthcare, Incyte Corporation, Loxo Oncology/Eli Lilly, MedImmune, Novartis, QED Therapeutics, Relay Therapeutics, Daiichi-Sankyo, and R-PharmUS; and other relationship with Medscape.
Ethical statement and consent
Authors obtained informed consent from the patient for sharing information related to the case including clinical and imaging data which have been de-identified during manuscript writing.
- 1. Phase 1 clinical trials for sarcomas: the cutting edge. Curr Opin Oncol. 2011; 23:352–60. https://doi.org/10.1097/CCO.0b013e3283477a94. PMID:21519259
- 2. Ewing’s sarcoma: standard and experimental treatment options. Curr Treat Options Oncol. 2009; 10:126–40. https://doi.org/10.1007/s11864-009-0104-6. PMID:19533369
- 3. Alpha Particle Radium 223 Dichloride in High-risk Osteosarcoma: A Phase I Dose Escalation Trial. Clin Cancer Res. 2019; 25:3802–10. https://doi.org/10.1158/1078-0432.CCR-18-3964. PMID:30733229
- 4. Ewing’s sarcoma: overcoming the therapeutic plateau. Discov Med. 2012; 13:405–15. PMID:22742646
- 5. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin Proc. 2012; 87:475–87. https://doi.org/10.1016/j.mayocp.2012.01.015. PMID:22560526
- 6. Clinical characteristics and outcomes of pediatric oncology patients with aggressive biology enrolled in phase I clinical trials designed for adults: the university of Texas MD anderson cancer center experience. Oncoscience. 2014; 1:522–30. https://doi.org/10.18632/oncoscience.68. PMID:25587555
- 7. Theranostic Profiling for Actionable Aberrations in Advanced High Risk Osteosarcoma with Aggressive Biology Reveals High Molecular Diversity: The Human Fingerprint Hypothesis. Oncoscience. 2014; 1:167–79. https://doi.org/10.18632/oncoscience.21. PMID:25126591
- 8. Drug review: Pazopanib. Jpn J Clin Oncol. 2018; 48:503–13. https://doi.org/10.1093/jjco/hyy053. PMID:29684209
- 9. Pazopanib for second recurrence of osteosarcoma in pediatric patients. Pediatr Int. 2017; 59:937–38. https://doi.org/10.1111/ped.13307. PMID:28685501
- 10. An mTOR and VEGFR inhibitor combination arrests a doxorubicin resistant lung metastatic osteosarcoma in a PDOX mouse model. Sci Rep. 2021; 11:8583. https://doi.org/10.1038/s41598-021-87553-9. PMID:33883561
- 11. Pazopanib and Trametinib as a Synergistic Strategy against Osteosarcoma: Preclinical Activity and Molecular Insights. Cancers (Basel). 2020; 12:1519. https://doi.org/10.3390/cancers12061519. PMID:32531992
- 12. Initial testing of the multitargeted kinase inhibitor pazopanib by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2012; 59:586–88. https://doi.org/10.1002/pbc.24016. PMID:22190407
- 13. Metronomic oral topotecan with pazopanib is an active antiangiogenic regimen in mouse models of aggressive pediatric solid tumor. Clin Cancer Res. 2011; 17:5656–67. https://doi.org/10.1158/1078-0432.CCR-11-0078. PMID:21788355
- 14. Phase II study of pazopanib with oral topotecan in patients with metastatic and non-resectable soft tissue and bone sarcomas. Br J Cancer. 2021; 125:528–33. https://doi.org/10.1038/s41416-021-01448-0. PMID:34050255
- 15. Pazopanib in Patients with Osteosarcoma Metastatic to the Lung: Phase 2 Study Results and the Lessons for Tumor Measurement. J Oncol. 2022; 2022:3691025. https://doi.org/10.1155/2022/3691025. PMID:35075361
- 16. Pazopanib in the Treatment of Bone Sarcomas: Clinical Experience. Transl Oncol. 2020; 13:295–99. https://doi.org/10.1016/j.tranon.2019.12.001. PMID:31875575
- 17. Response to Pazopanib in Patients With Relapsed Osteosarcoma. J Pediatr Hematol Oncol. 2020; 42:e254–57. https://doi.org/10.1097/MPH.0000000000001375. PMID:30531600
- 18. Pazopanib maintenance therapy after tandem high-dose chemotherapy for disseminated Ewing sarcoma. Int Cancer Conf J. 2019; 8:95–100. https://doi.org/10.1007/s13691-019-00362-w. PMID:31218182
- 19. Real-World Experiences with Pazopanib in Patients with Advanced Soft Tissue and Bone Sarcoma in Northern California. Med Sci (Basel). 2019; 7:48. https://doi.org/10.3390/medsci7030048. PMID:30889920
- 20. Pazopanib Confers a Progression-free Survival in a Patient with Ewing’s Sarcoma/Primitive Neuroectodermal Tumor of the Lung. Intern Med. 2019; 58:1335–39. https://doi.org/10.2169/internalmedicine.1549-18. PMID:30626819
- 21. Pazopanib in relapsed osteosarcoma patients: report on 15 cases. Acta Oncol. 2019; 58:124–28. https://doi.org/10.1080/0284186X.2018.1503714. PMID:30207179
- 22. The Successful Treatment of Metastatic Extraosseous Ewing Sarcoma with Pazopanib. Intern Med. 2018; 57:2753–57. https://doi.org/10.2169/internalmedicine.9879-17. PMID:29780156
- 23. Malignant Ewing-Like Neoplasm With an EWSR1-KLF15 Fusion: At the Crossroads of a Myoepithelial Carcinoma and a Ewing-Like Sarcoma. A Case Report With Treatment Options. Int J Surg Pathol. 2018; 26:440–47. https://doi.org/10.1177/1066896918755009. PMID:29390927
- 24. Zoledronic acid in metastatic osteosarcoma: encouraging progression free survival in four consecutive patients. Clin Sarcoma Res. 2016; 6:6. https://doi.org/10.1186/s13569-016-0046-2. PMID:27127605
- 25. Off-label use of targeted therapies in osteosarcomas: data from the French registry OUTC’S (Observatoire de l’Utilisation des Thérapies Ciblées dans les Sarcomes). BMC Cancer. 2015; 15:854. https://doi.org/10.1186/s12885-015-1894-5. PMID:26541413
- 26. Clinical Activity of Pazopanib in Metastatic Extraosseous Ewing Sarcoma. Rare Tumors. 2015; 7:5992. https://doi.org/10.4081/rt.2015.5992. PMID:26266019
- 27. Response of refractory Ewing sarcoma to pazopanib. Acta Oncol. 2015; 54:1063–64. https://doi.org/10.3109/0284186X.2014.971938. PMID:25345493
- 28. Pazopanib in metastatic osteosarcoma: significant clinical response in three consecutive patients. Acta Oncol. 2014; 53:1451–54. https://doi.org/10.3109/0284186X.2014.948062. PMID:25143189
- 29. Pazopanib for recurrent extraosseous Ewing’s sarcoma of the retroperitoneum. Int J Urol. 2014; 21:1183–84. https://doi.org/10.1111/iju.12546. PMID:25040171
- 30. Personalized comprehensive molecular profiling of high risk osteosarcoma: Implications and limitations for precision medicine. Oncotarget. 2015; 6:40642–54. https://doi.org/10.18632/oncotarget.5841. PMID:26510912
- 31. Examining Stripes on a Herd of Zebras: Impact of Genomic Matching for Ultrarare Sarcomas in Phase 1 Clinical Trials (SAMBA 102). Clin Cancer Res. 2023; 29:401–9. https://doi.org/10.1158/1078-0432.CCR-22-2509. PMID:36288393
- 32. EWSR1-The Most Common Rearranged Gene in Soft Tissue Lesions, Which Also Occurs in Different Bone Lesions: An Updated Review. Diagnostics (Basel). 2021; 11:1093. https://doi.org/10.3390/diagnostics11061093. PMID:34203801
- 33. EWSR1-NFATC2 Translocation-associated Sarcoma Clinicopathologic Findings in a Rare Aggressive Primary Bone or Soft Tissue Tumor. Am J Surg Pathol. 2019; 43:1112–22. https://doi.org/10.1097/PAS.0000000000001260. PMID:30994538
- 34. Multiscale-omic assessment of EWSR1-NFATc2 fusion positive sarcomas identifies the mTOR pathway as a potential therapeutic target. NPJ Precis Oncol. 2021; 5:43. https://doi.org/10.1038/s41698-021-00177-0. PMID:34021224
- 35. Targeting mTOR as a Cancer Therapy: Recent Advances in Natural Bioactive Compounds and Immunotherapy. Cancers (Basel). 2022; 14:5520. https://doi.org/10.3390/cancers14225520. PMID:36428613
- 36. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol. 2018; 15:273–91. https://doi.org/10.1038/nrclinonc.2018.28. PMID:29508857
- 37. Topoisomerase I amplification in melanoma is associated with more advanced tumours and poor prognosis. Pigment Cell Melanoma Res. 2010; 23:542–53. https://doi.org/10.1111/j.1755-148X.2010.00720.x. PMID:20465595
- 38. Soft tissue round cell sarcoma of the abdominal wall, with EWSR1-non-ETS fusion (EWSR1-NFATC2 sarcoma): A case report and literature review emphasizing its clinical features. J Orthop Sci. 2022. [Epub ahead of print]. https://doi.org/10.1016/j.jos.2022.02.009. PMID:35430129
- 39. Implementation of Copy Number Variations-Based Diagnostics in Morphologically Challenging EWSR1/FUS::NFATC2 Neoplasms of the Bone and Soft Tissue. Int J Mol Sci. 2022; 23:16196. https://doi.org/10.3390/ijms232416196. PMID:36555836
- 40. A unique epithelioid vascular neoplasm of bone characterized by EWSR1/FUS-NFATC1/2 fusions. Genes Chromosomes Cancer. 2021; 60:762–71. https://doi.org/10.1002/gcc.22984. PMID:34310785
- 41. NKX3-1 Is a Useful Immunohistochemical Marker of EWSR1-NFATC2 Sarcoma and Mesenchymal Chondrosarcoma. Am J Surg Pathol. 2020; 44:719–28. https://doi.org/10.1097/PAS.0000000000001441. PMID:31972596
- 42. NFATc2-rearranged sarcomas: clinicopathologic, molecular, and cytogenetic study of 7 cases with evidence of AGGRECAN as a novel diagnostic marker. Mod Pathol. 2020; 33:1930–44. https://doi.org/10.1038/s41379-020-0542-z. PMID:32327700
- 43. The clinical heterogeneity of round cell sarcomas with EWSR1/FUS gene fusions: Impact of gene fusion type on clinical features and outcome. Genes Chromosomes Cancer. 2020; 59:525–34. https://doi.org/10.1002/gcc.22857. PMID:32362012
- 44. Bone Sarcoma With EWSR1-NFATC2 Fusion: Sarcoma With Varied Morphology and Amplification of Fusion Gene Distinct From Ewing Sarcoma. Int J Surg Pathol. 2019; 27:561–67. https://doi.org/10.1177/1066896919827093. PMID:30714449
- 45. DNA methylation profiling distinguishes Ewing-like sarcoma with EWSR1-NFATc2 fusion from Ewing sarcoma. J Cancer Res Clin Oncol. 2019; 145:1273–81. https://doi.org/10.1007/s00432-019-02895-2. PMID:30895378
- 46. EWSR1-NFATC2 and FUS-NFATC2 Gene Fusion-Associated Mesenchymal Tumors: Clinicopathologic Correlation and Literature Review. Sarcoma. 2019; 2019:9386390. https://doi.org/10.1155/2019/9386390. PMID:31049020
- 47. EWSR1/FUS-NFATc2 rearranged round cell sarcoma: clinicopathological series of 4 cases and literature review. Hum Pathol. 2019; 90:45–53. https://doi.org/10.1016/j.humpath.2019.05.001. PMID:31078563
- 48. Transcriptomic definition of molecular subgroups of small round cell sarcomas. J Pathol. 2018; 245:29–40. https://doi.org/10.1002/path.5053. PMID:29431183
- 49. Review with novel markers facilitates precise categorization of 41 cases of diagnostically challenging, “undifferentiated small round cell tumors”. A clinicopathologic, immunophenotypic and molecular analysis. Ann Diagn Pathol. 2018; 34:1–12. https://doi.org/10.1016/j.anndiagpath.2017.11.011. PMID:29661713
- 50. PAX7 immunohistochemical evaluation of Ewing sarcoma and other small round cell tumours. Histopathology. 2018; 73:645–52. https://doi.org/10.1111/his.13689. PMID:29920735
- 51. EWSR1-NFATC2 gene fusion in a soft tissue tumor with epithelioid round cell morphology and abundant stroma: a case report and review of the literature. Hum Pathol. 2018; 81:281–90. https://doi.org/10.1016/j.humpath.2018.03.020. PMID:29626598
- 52. EWSR1 fusion proteins mediate PAX7 expression in Ewing sarcoma. Mod Pathol. 2017; 30:1312–20. https://doi.org/10.1038/modpathol.2017.49. PMID:28643791
- 53. Robust diagnosis of Ewing sarcoma by immunohistochemical detection of super-enhancer-driven EWSR1-ETS targets. Oncotarget. 2018; 9:1587–601. https://doi.org/10.18632/oncotarget.20098. PMID:29416716
- 54. Kinkor Z, Vaneček T, Svajdler M Jr, Mukenšnabl P, Veselý K, Baxa J, Kokavec M. Cesk Patol. 2014; 50:87–91. PMID:24758504
- 55. Malignant round cell tumor of bone with EWSR1-NFATC2 gene fusion. Virchows Arch. 2014; 465:233–39. https://doi.org/10.1007/s00428-014-1613-7. PMID:24993903
- 56. Expression of ERG, an Ets family transcription factor, identifies ERG-rearranged Ewing sarcoma. Mod Pathol. 2012; 25:1378–83. https://doi.org/10.1038/modpathol.2012.97. PMID:22766791
- 57. The NFATc2 gene is involved in a novel cloned translocation in a Ewing sarcoma variant that couples its function in immunology to oncology. Clin Cancer Res. 2009; 15:2259–68. https://doi.org/10.1158/1078-0432.CCR-08-2184. PMID:19318479
- 58. Malignant fibrous histiocytoma and fibrosarcoma of bone: a re-assessment in the light of currently employed morphological, immunohistochemical and molecular approaches. Virchows Arch. 2012; 461:561–70. https://doi.org/10.1007/s00428-012-1306-z. PMID:23001328
- 59. A benign vascular tumor with a new fusion gene: EWSR1-NFATC1 in hemangioma of the bone. Am J Surg Pathol. 2013; 37:613–16. https://doi.org/10.1097/PAS.0b013e31827ae13b. PMID:23480895
- 60. Detecting disease-defining gene fusions in unclassified round cell sarcomas using anchored multiplex PCR/targeted RNA next-generation sequencing-Molecular and clinicopathological characterization of 16 cases. Genes Chromosomes Cancer. 2019; 58:713–22. https://doi.org/10.1002/gcc.22763. PMID:31033080
- 61. Identification of EWSR1-NFATC2 fusion in simple bone cysts. Histopathology. 2021; 78:849–56. https://doi.org/10.1111/his.14314. PMID:33316098
- 62. Expanding the Spectrum of EWSR1-NFATC2-rearranged Benign Tumors: A Common Genomic Abnormality in Vascular Malformation/Hemangioma and Simple Bone Cyst. Am J Surg Pathol. 2021; 45:1669–81. https://doi.org/10.1097/PAS.0000000000001748. PMID:34081036
- 63. Pižem J, Šekoranja D, Matjašič A, Zupan A, Boštjančič E, Limpel Novak KA, Salapura V, Mavčič B, Gazič B, Dimnik K. Virchows Arch. 2021; 479:795–802. https://doi.org/10.1007/s00428-021-03130-5. PMID:34089379
- 64. Skeletal EWSR1-NFATC2 sarcoma previously diagnosed as Ewing-like adamantinoma: A case report and literature review emphasizing its unique radiological features. Pathol Int. 2021; 71:614–20. https://doi.org/10.1111/pin.13135. PMID:34192825
- 65. EWS/Fli-1 chimeric fusion gene upregulates vascular endothelial growth factor-A. Int J Cancer. 2010; 126:2790–98. https://doi.org/10.1002/ijc.24781. PMID:19642105
- 66. EWS/FLI1 regulates tumor angiogenesis in Ewing’s sarcoma via suppression of thrombospondins. Cancer Res. 2007; 67:6675–84. https://doi.org/10.1158/0008-5472.CAN-06-4140. PMID:17638877
- 67. PI3K/AKT/mTOR Pathway in Angiogenesis. Front Mol Neurosci. 2011; 4:51. https://doi.org/10.3389/fnmol.2011.00051. PMID:22144946
- 68. Activity of c-Met/ALK Inhibitor Crizotinib and Multi-Kinase VEGF Inhibitor Pazopanib in Metastatic Gastrointestinal Neuroectodermal Tumor Harboring EWSR1-CREB1 Fusion. Oncology. 2016; 91:348–53. https://doi.org/10.1159/000449204. PMID:27764830
- 69. Establishment of a novel clear cell sarcoma cell line (Hewga-CCS), and investigation of the antitumor effects of pazopanib on Hewga-CCS. BMC Cancer. 2014; 14:455. https://doi.org/10.1186/1471-2407-14-455. PMID:24946937
