Oncoscience
Non-selective beta blockers inhibit angiosarcoma cell viability and increase progression free- and overall-survival in patients diagnosed with metastatic angiosarcoma
Clarissa N. Amaya1, Mariah Perkins2, Andres Belmont3, Connie Herrera3, Arezo Nasrazadani3, Alejandro Vargas3, Thuraieh Khayou1, Alexa Montoya1,4, Yessenia Ballou1, Dana Galvan3, Alexandria Rivas1, Steven Rains1, Luv Patel3, Vanessa Ortega1, Christopher Lopez1, William Chow5, Erin B. Dickerson6,7 and Brad A. Bryan1,3
1
Department of Biomedical Sciences, Texas Tech University Health Sciences Center, El Paso, TX, USA
2
Department of Biochemistry, Baylor University, Waco, TX, USA
3
Paul L. Foster School of Medicine, Texas Tech University Health Sciences Center, El Paso, TX, USA
4
Department of Biology, University of Texas, El Paso, TX, USA
5
Mohs Micrographic Surgery and Cutaneous Oncology, San Leandro, CA, USA
6
Department of Veterinary Clinical Sciences, University of Minnesota, St. Paul, MN, USA
7
Masonic Cancer Center, University of Minnesota, Minneapolis, MN, USA
Correspondence to: Brad A. Bryan, email:[email protected]
Keywords: angiosarcoma, propranolol, beta blocker, sarcoma
Received: January 09, 2018
Accepted: March 02, 2018
Published: April 29, 2018
Copyright: Amaya et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
Patients with metastatic angiosarcoma undergoing chemotherapy, radiation, and/or surgery experience a median progression free survival of less than 6 months and a median overall survival of less than 12 months. Given the aggressive nature of this cancer, angiosarcoma clinical responses to chemotherapy or targeted therapeutics are generally very poor. Inhibition of beta adrenergic receptor (β-AR) signaling has recently been shown to decrease angiosarcoma tumor cell viability, abrogate tumor growth in mouse models, and decrease proliferation rates in preclinical and clinical settings. In the current study we used cell and animal tumor models to show that β-AR antagonism abrogates mitogenic signaling and reduces angiosarcoma tumor cell viability, and these molecular alterations translated into patient tumors. We demonstrated that non-selective β-AR antagonists are superior to selective β-AR antagonists at inhibiting angiosarcoma cell viability. A prospective analysis of non-selective β-AR antagonists in a single arm clinical study of metastatic angiosarcoma patients revealed that incorporation of either propranolol or carvedilol into patients’ treatment regimens leads to a median progression free and overall survival of 9 and 36 months, respectively. These data suggest that incorporation of non-selective β-AR antagonists into existing therapies against metastatic angiosarcoma can enhance clinical outcomes.
INTRODUCTION
Angiosarcomas are rare vascular tumors of aberrant endothelial histology that exhibit an aggressive and often highly lethal natural course. These tumors can manifest at various sites on the body, but are most frequent in the skin, soft tissue, liver, breast, spleen, bone, or heart [1, 2]. Metastatic angiosarcoma patients face a dismal median progression free survival (PFS) of 3 to 6 months and median overall survival (OS) of 3 to 12 months [3-8]. Overall treatment response rates to chemotherapy or targeted therapeutics in patients with metastatic disease are generally between 10-20% [6, 7, 9-11], and there are no established treatment guidelines for metastatic disease. Because of the poor prognosis of patients with metastatic angiosarcoma, novel therapies that improve outcome are desperately needed.
Several recent studies have reported beta adrenergic receptor (β-AR) expression across diverse tumor types [12, 23], and a growing body of literature suggests a role for β-AR signaling in regulating multiple hallmarks of benign and malignant tumors [24, 28]. Inhibition of β-AR signaling by receptor antagonists (beta blockers) has become the gold standard treatment for the benign vascular tumor infantile hemangioma [27, 29] in part through disrupting tumor cell proliferation [30]. By translating these findings to malignant angiosarcomas, we showed that propranolol exhibits selective cytotoxicity and tumor suppressive ability against these tumors [21]. Clinical translation of propranolol in combination with chemotherapy drugs has generated overwhelming clinical responses in all reports to date of its use in angiosarcoma patients [31, 38]. Among these studies, Pasquier et al. combined propranolol and the microtubule-targeting agent vinblastine in seven patients with advanced angiosarcomas, and showed that incorporation of propranolol into the chemotherapy regimen leads to a median PFS and median OS at 11 and 16 months, respectively [36]. Collectively, the clinical effectiveness of propranolol against angiosarcomas recently led to its Orphan Drug Designation by the European Medicines Agency (EMA) for use against soft tissue sarcomas.
In addition to vascular tumors, β-AR antagonists have shown preclinical efficacy against a variety of malignant tumors including breast cancer [10, 15, 39-41], neuroblastoma [42], and melanoma [43-48]. Retrospective analyses of patient data have also revealed a strong correlation between reduced tumor progression, metastasis, and mortality, and the use of β-AR antagonists in breast, ovarian, and prostate cancer patients, as well as melanoma patients [15, 49-58]. Prospective clinical studies have reported that propranolol use results in a reduced breast tumor proliferation rates [15], and, when combined with COX-2 inhibitors, β-AR antagonists inhibit multiple cellular and molecular pathways related to metastasis and disease recurrence [59].
In the current study, we evaluated the efficacy of selective (targeting only one β-AR) and non-selective (targeting multiple β-ARs) beta blockers using in vitro angiosarcoma models. We then quantified clinical outcomes of patients with metastatic disease prescribed non-selective beta blockers in combination with standard treatment regimens.
RESULTS
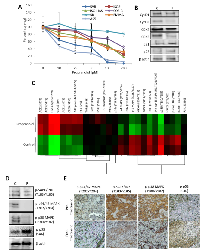
β-AR antagonism disrupts sarcoma cell viability. We have previously shown that β-AR antagonists inhibit the viability of angiosarcoma cells and limit tumor progression [21, 32, 36]. We expanded these findings to additional angiosarcoma and hemangiosarcoma cells lines, revealing dose-dependent decreases in cell viability in response to propranolol for all cell lines with the exception of SB-HSA, a hemangiosarcoma line (Figure 1A). Treatment of SVR cells with propranolol reduced the expression of cyclins and cyclin dependent kinases, and increased the expression of cyclin dependent kinase inhibitors (Figure 1B), confirming the impact of propranolol at the molecular level. We then used antibody arrays to assess signaling alterations induced after 1 hour treatment with propranolol, revealing activation of cell stress/survival mediators including AKT and p53, and inhibition of multiple MAPK mediators such as p42, p44, JNK, and p38 (Figure 1C). A subset of these signaling alterations was confirmed in mice bearing SVR angiosarcoma xenografts treated with 15 mg/kg propranolol for 15 minutes. A comparison with tumors from sham treated controls revealed decreased mitogenic p42/44, JNK/SAPK, and p38 phosphorylation and increased apoptotic p53 phosphorylation (Figure 1D). We confirmed these results in tumor samples obtained from an angiosarcoma patient taken before and one week after treatment with propranolol monotherapy. Evaluation of the expression of levels of phosphorylated p44/42, JNK/SAPK, and p38, as well as the apoptotic regulator p53 by IHC showed the levels of all three mitogenic regulators were markedly reduced following propranolol, while the level of phosphorylated p53 was increased (Figure 1E).
Non-selective β-AR antagonists are superior to selective β-AR antagonists for inhibiting angiosarcoma viability. Propranolol is a non-selective β-AR antagonist that classically inhibits both β1-AR and β2-AR. Our lab and others have previously shown that non-selective β-AR antagonists are superior to selective β-AR antagonists with regard to decreasing tumor cell proliferation and enhancing clinical outcome in breast, liver, and ovarian cancer [15, 57, 58]. To determine if a similar outcome could be observed in angiosarcoma models, we evaluated the viability of two angiosarcoma cell lines following treatment with propranolol, β1-AR selective antagonists (esmolol and atenolol), or β2-AR selective antagonists (butoxamine and ICI-118,551). β3-AR selective antagonists were not evaluated since selective antagonists targeting this receptor are not clinically available to patients. While moderate decreases in cell viability were observed following treatment of the angiosarcoma cells with the selective receptor antagonists, none of the selective antagonists were as effective as propranolol (Figure 2A & 2B). To determine if the observed difference was due to activation or inhibition of different cell signaling pathways, SVR cells were treated with non-selective or selective antagonists for 1 hour and mitogenic and survival signaling changes were evaluated using antibody arrays. Compared to control samples, the majority of the protein phosphorylation events were consistent between propranolol and the selective β-AR antagonists. The major exceptions to this involved propranolol-mediated upregulation of AKT and p53 phosphorylation, which did not occur upon treatment with the selective β–AR antagonists (Figure 2C).
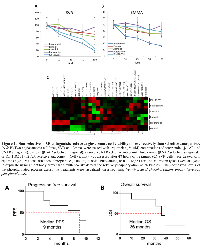
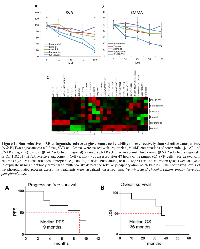
Non-selective β-AR antagonists extend progression free and overall survival in patients with metastatic angiosarcoma. Patients with an initial diagnosis of metastatic angiosarcoma were prescribed the non-selective beta blockers propranolol or carvedilol in addition to their anti-cancer treatment regimen. Six women and three men were included in this study, with a median age of 53.7 (range=34-75). Each patient was given propranolol (20 to 100 mg per day; n=8) or carvedilol (6.25 mg per day; n=1) in combination with treatment protocols, which varied by treating oncologist (Table 1). Patients treated with non-selective β-AR antagonists were chosen for this study based on the stronger efficacy observed in our preclinical models of angiosarcoma (Figure 1 and Figure 2). A single patient declined chemotherapy or radiation, and was only prescribed propranolol and supportive care. All patients were monitored between the years of 2013 to 2017. PFS and OS rates were improved in patients taking β-AR antagonists relative to the historical controls [4]. Specifically, administration of β−AR antagonists in combination with anti-cancer therapies resulted in a median PFS of 9 months verses previous reports of 3 to 6 months (Figure 3A), and a median OS of 36 months verses documented reports of 12 months (Figure 3B). The correlation coefficient between daily dose of propranolol and PFS was 0.25, suggesting a very weak/absent relationship between the doses of propranolol used in this study and clinical outcomes.
DISCUSSION
The data presented in this study demonstrate that non-selective β-AR antagonists effectively inhibit tumor cell viability and mitogenic signaling in angiosarcomas. Furthermore, incorporation of non-selective β-AR antagonists into the standard treatment of patients with metastatic angiosarcoma substantially increased PFS and OS compared to historical controls.
The influence of β-AR signaling on non-diseased and tumor cell proliferation rates was reported in the literature more than half a century ago [28]. We have previously reported in angiosarcoma and breast cancer patient case studies that neoadjuvant administration of propranolol alone reduced the tumor proliferative index based on quantification of tumor Ki-67 levels [15, 32]. With the exception of the propranolol-resistant SB cell line, our current data demonstrates that concentrations of propranolol as low as 10 µM reduces angiosarcoma cell viability between 6% to 53% compared to controls, with increasingly stronger anti-proliferative affects observed at elevated concentrations of the drug. We validated these findings at the molecular level using in vitro assays, a xenograft tumor model, and patient tumor analysis to demonstrate that propranolol consistently reduces mitogenic signaling and increases apoptotic signaling in angiosarcomas. While our results show that β-AR antagonists disrupt tumor cell function, the dramatic anti-tumor responses observed following administration of β-AR antagonists in angiosarcoma patients may also be due, at least in part, to effects on cells within the tumor stroma. For example, β-AR stimulation increases cellular transformation of fibroblast cells in soft agar [74], suggesting that catecholamine signaling could directly influence tumor stromal behavior. β-AR antagonists also potentiate the anti-angiogenic effects of chemotherapy agents [36] and suppress cell cycle progression and chemotactic motility in endothelial cells [75], suggesting an anti-angiogenic function for these drugs. Catecholamine stimulation of β-ARs disrupts immune cell differentiation, impairs the cytotoxicity of natural killer cells and expansion of memory CD8+ T-cells, and alters T-helper cell responses [76-83]. Recently, propranolol has been shown to influence the activity of the immune system where it facilities the conversion of tumors to an immunologically active tumor microenvironment with increased T-cell infiltration and decreased immunosuppressive PD1 expression [44], suggesting that adrenergic antagonists may be an effective adjuvant to immunotherapy.
Retrospective studies in breast, ovarian, and liver cancer suggest that non-selective β−AR antagonists such as propranolol are more effective against tumors than selective receptor antagonists, which target single β-AR receptors [15, 57, 58]. The current data in angiosarcoma validates previous findings from other cancer types by showing that propranolol more effectively reduces tumor cell viability than β1- or β2-AR-selective antagonists. At the molecular level, non-selective and selective antagonists similarly affect post-translational modifications of mitogenic regulators; however, only propranolol induced phosphorylation of AKT and p53 proteins, suggesting a differential regulation of survival/apoptotic proteins between receptor-selective and non-selective drugs. While all three β-AR receptors are expressed across a variety of cancer types [23], including angiosarcoma [30, 32], the individual contribution each receptor makes to the overall oncogenic processes is not known. It is possible that selective antagonism of a single β-AR is compensated by other expressed β-ARs, thus explaining the insufficiency of selective antagonists to recapitulate the anti-proliferative effects of non-selective antagonists.The data presented in this study revealed that incorporation of non-selective β-AR antagonism into standard treatment regimens increased PFS and OS from approximately 3 to 6 months and 24 to 33 months, respectively, relative to reported results from several key clinical studies that incorporated only chemotherapy, radiation, and/or surgery [3-8]. The efficacy reported in the current study corroborates data previously published by Pasquier and colleagues showing increased PFS and OS for angiosarcoma patients taking propranolol in combination with chemotherapy [36]. We did not observe a significant correlation between the dose of antagonist and clinical outcome based on PFS, therefore future studies with larger patient cohorts will be needed to optimize dosage. While propranolol led to efficacy when combined with other treatment modalities, we were very surprised to see that when used as a single agent propranolol resulted in sustained disease regression for a patient who declined chemotherapy, radiation, and surgery. While this single instance in no way suggests that patients should opt for adrenergic antagonists in lieu of other established treatment options against metastatic angiosarcoma, this limited observation raises the question if these drugs could be used to extend survival in patients who decline standard treatment and opt for only supportive care.
MATERIALS AND METHODS
Cell culture and chemicals. SVR angiosarcoma [60, 61] (ATCC #CRL-2280) and EMMA hemangiosarcoma cells [62] were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin (P/S). COSB hemangiosarcoma [63], SB-HSA hemangiosarcoma cells [64], ISOS-1 angiosarcoma [65], ISO-HAS angiosarcoma [66], and AS5 angiosarcoma cells [67] were grown in EBM-2 Basal Medium (Lonza #CC-3156) supplemented with EGM2 Bulletkit (Lonza #CC-4176). All cells were cultured in 37°C water-jacketed CO2 incubators maintained for gas, temperature, and humidity. The Sex Primary Metastases Treatment Antagonist Dose/day F Breast Skull Doxorubicin, Cyclophosphamide, Radiotherapy Propranolol 60 mg M Spleen Bone marrow, liver Doxorubicin, Radiotherapy, Surgery Carvedilol 6.25 mg F Breast Lymph nodes, chest cavity Paclitaxel, Ifosfamide, Carboplatin Propranolol 40 mg M Pleura Lymph nodes, bone Doxorubicin Propranolol 100 mg F Breast Lungs Paclitaxel, Cyclophosphamide Propranolol 60 mg M Liver Spleen, Lungs Doxorubicin, Radiotherapy Propranolol 40 mg F Breast Liver Doxorubicin, Gemcitabine Propranolol 20 mg F Breast Brain, Bone, Lungs, Maxillary Sinus, Thigh Doxorubicin, Ifosfamide, Paclitaxel, Gemcitabine, Radiotherapy Propranolol 40 mg F Cardiac Lungs, Liver None Propranolol 40 mg non-selective β-AR antagonist propranolol, the β1-AR selective antagonist esmolol, the β1-AR selective antagonist atenolol, the β2-AR selective antagonist butoxamine, and the β2-AR selective antagonist ICI-118,551 were used at the concentration and timing as indicated for each experiment.
Viability assays. To measure viability, cells were plated in 24 well plates at 0.3 x 106 cells/well, treated as indicated for each experiment, and cell viability was monitored over a 48 hour time course using time lapse microscopy with a Nikon Biostation CT robot. Cell number was manually counted at t=0 hours and t=48 hours. The difference in cell number was used to reflect the viability change for each condition. At least three biological replicates were performed for every cell line, with at least four technical replicates per assay. Data is presented as the average of the technical replicates for a single biological replicate.
Immunoblotting. Lysates from cell culture or tumor tissue were collected as indicated for each experiment, subjected to SDS-PAGE, and transferred to polyvinylidene fluoride membranes using the Trans-Blot Turbo Transfer System (Bio-Rad). Membranes were blocked in tris buffered saline plus 3% bovine serum albumin and 0.05% Tween-20, and incubated with the following antibodies as indicated for each experiment: anti-phospho-p38 (T180/Y182) (Cell Signaling #4511), anti-phospho-SAPK/JNK (T183/Y185) (Cell Signaling #4668), anti-phospho-p44/42 (T202/Y204) (Cell Signaling #4370), anti-phospho-p53 (S46) (Cell Signaling #2521), anti-cyclin D1 (Cell Signaling #2978), anti-cyclin D3 (Cell Signaling #2936), anti-CDK4 (Cell Signaling #12790), anti-CDK6 (Cell Signaling #13331), anti-p21 (Cell Signaling #2947), anti-p27 (Cell Signaling #3686), and anti-β actin (Santa Cruz Biotech #sc8432). Each primary antibody was detected with an appropriate 1:1000 HRP-conjugated secondary antibody, subjected to Supersignal West Dura Extended Duration Substrate (ThermoFisher Scientific #34075), and digitally captured using a GE Image Quant Las4000 imaging system.
Antibody array. The Phospho-Mitogen-Activated Protein Kinase (MAPK) Antibody Array (R&D Systems #ARY002B) was performed according to the manufacturer’s instructions using SVR cell lysates. Densitometry of each antibody array signal was performed using ImageJ software. Reference spots on each array were used to normalize the pixel densities. The numerical protein expression data was normalized, and centroid linkage based on an uncentered correlation similarity metric was performed using Gene Cluster 3.0 software. Heatmaps were generated using JavaTreeView software.
Animal models. Animal experiments were performed in accordance to Texas Tech University Health Sciences Center Institutional Animal Care and Use Committee regulations for the care and use of animals in experimental procedures, and all efforts were made to minimize suffering. Mice were housed 4 per cage in a temperature-controlled animal facility on a 12h-12h light-dark cycle. Animals had free access to mouse chow and water. Xenograft angiosarcoma tumor models were generated via subcutaneous injection of 1x105 SVR cells into the dorsolateral flank of 6 week old male J/Nu mice (N=5 mice per experimental condition). Tumors were allowed to grow until ~1cm3, and injected with saline sham or 15 mg/kg propranolol for 15 minutes. Tumor tissue was collected and immediately flash frozen in liquid nitrogen. Tissue lysates were pooled prior to use in immunoblotting.
Immunohistochemistry (IHC). IHC was performed on tumor tissue samples collected from an angiosarcoma patient before and after one week of treatment with propranolol [32]. The following antibodies were used: anti-p-p44/42 (Thr202/Tyr204) (Cell Signaling #4370), anti-p-SAPK/JNK (Thr183/Tyr185) (Cell Signaling #4668), anti-p-p38 (T180/Y182) (Abcam #4822), and anti-phospho-p53 (S46) (Cell Signaling #2521). Antigenicity was detected using the mouse and rabbit Specific HRP/DAB (ABC) Detection IHC Kit (Abcam #ab64264) according to the manufacturer’s protocol.
Clinical study. This clinical study was approved by the Texas Tech University Health Sciences Center Institutional Review Board (IRB# E17109). As angiosarcoma is a very rare tumor and most individual clinical sites do not see enough patients with this tumor type to accommodate a clinical trial, we recruited nine angiosarcoma patients (six women and three men) with a diagnosis of metastatic disease through the Angiosarcoma Awareness patient support group on Facebook. The median age for this cohort was 55 years with a range from 34 to 75 years. Similar recruitment strategies using social media have been effective for a number of rare diseases where any single treatment center would lack the patient volume necessary to conduct a clinical study [68-73]. PFS and OS were obtained for each patient, and the data were compared to historical controls (25 years of data describing clinical outcomes in angiosarcoma patients treated at the Memorial Sloan Kettering Cancer Center) [4]. Kaplan-Meier plots were generated and statistical analysis was performed using GraphPad Prism 7.03. The Pearson’s correlation coefficient for PFS and dose of propranolol was calculated using Microsoft Excel.
CONCLUSION
Due to the poor prognosis of metastatic angiosarcoma patients, many are faced with the decision to undergo chemotherapy, radiation, and/or surgery or simply accept supportive care—choices that generally lead to similar outcomes. The data presented in this study suggest that incorporation of non-selective β-AR antagonists into a variety of standard treatment regimens enhances PFS and OS in patients with metastatic angiosarcoma. Use of inexpensive and relatively safe non-selective β-AR antagonists along with established treatments against angiosarcoma could improve prognoses for metastatic patients.
FUNDING
This work was supported by grants from Angiosarcoma Awareness Foundation (BAB) and Sarcoma Foundation of America (BAB and EBD).
- 1. Angiosarcoma. Lancet Oncol. 2010; 11: 983-91. https://doi.org/10.1016/S1470-2045(10)70023-1. [PubMed].
- 2. Primary and secondary angiosarcoma of the breast: the Mayo Clinic experience. J Surg Oncol. 2010; 101: 401-7. https://doi.org/10.1002/jso.21497. [PubMed].
- 3. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014; 37: 473-9. https://doi.org/10.1097/COC.0b013e31827e4e7b. [PubMed].
- 4. Outcomes of Systemic Therapy for Patients with Metastatic Angiosarcoma. Oncology. 2015; 89: 205-14. https://doi.org/10.1159/000381917. [PubMed].
- 5. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer. 2012; 118: 3330-6. https://doi.org/10.1002/cncr.26599. [PubMed].
- 6. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008; 26: 5269-74. https://doi.org/10.1200/JCO.2008.17.3146. [PubMed].
- 7. Testing new regimens in patients with advanced soft tissue sarcoma: analysis of publications from the last 10 years. Ann Oncol. 2011; 22: 1266-72. https://doi.org/10.1093/annonc/mdq608. [PubMed].
- 8. Clinical and diagnostic features of angiosarcoma with pulmonary metastases: A retrospective observational study. Medicine (Baltimore). 2017; 96: e8033. https://doi.org/10.1097/MD.0000000000008033. [PubMed].
- 9. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013; 24: 257-63. https://doi.org/10.1093/annonc/mds237. [PubMed].
- 10. Carvedilol suppresses migration and invasion of malignant breast cells by inactivating Src involving cAMP/PKA and PKCdelta signaling pathway. J Cancer Res Ther. 2014; 10: 998-1003. https://doi.org/10.4103/0973-1482.137664. [PubMed].
- 11. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009; 27: 3133-40. https://doi.org/10.1200/JCO.2008.20.4495. [PubMed].
- 12. Chen D, Xing W, Hong J, Wang M, Huang Y, Zhu C, Yuan Y, Zeng W. The beta2-adrenergic receptor is a potential prognostic biomarker for human hepatocellular carcinoma after curative resection. Ann Surg Oncol. 2012; 19: 3556-65. https://doi.org/10.1245/s10434-012-2396-1. [PubMed].
- 13. Chisholm KM, Chang KW, Truong MT, Kwok S, West RB, Heerema-McKenney AE. beta-Adrenergic receptor expression in vascular tumors. Mod Pathol. 2012; 25: 1446-51. https://doi.org/10.1038/modpathol.2012.108. [PubMed].
- 14. Association of alpha2a and beta2 adrenoceptor expression with clinical outcome in breast cancer. Curr Med Res Opin. 2014; 30: 1337-44. https://doi.org/10.1185/03007995.2014.890928. [PubMed].
- 15. Montoya A, Amaya CN, Belmont A, Diab N, Trevino R, Villanueva G, Rains S, Sanchez LA, Badri N, Otoukesh S, Khammanivong A, Liss D, Baca ST, et al. Use of non-selective beta-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget. 2017; 8: 6446-60. https://doi.org/10.18632/oncotarget.14119. [PubMed].
- 16. beta-adrenoceptors are upregulated in human melanoma and their activation releases protumorigenic cytokines and metalloproteases in melanoma cell lines. Lab Invest. 2013; 93: 279-90. https://doi.org/10.1038/labinvest.2012.175. [PubMed].
- 17. Alpha- and beta-adrenergic receptor (AR) protein expression is associated with poor clinical outcome in breast cancer: an immunohistochemical study. Breast Cancer Res Treat. 2011; 130: 457-63. https://doi.org/10.1007/s10549-011-1371-z. [PubMed].
- 18. Schuller HM, Plummer HK, 3rd, Bochsler PN, Dudric P, Bell JL, Harris RE. Co-expression of beta-adrenergic receptors and cyclooxygenase-2 in pulmonary adenocarcinoma. Int J Oncol. 2001; 19: 445-9. doi. [PubMed].
- 19. Expression of beta2adrenergic receptor in oral squamous cell carcinoma. J Oral Pathol Med. 2009; 38: 371-6. https://doi.org/10.1111/j.1600-0714.2008.00691.x. [PubMed].
- 20. Prognostic significance of beta2-adrenergic receptor expression in malignant melanoma. Tumour Biol. 2016; 37: 5971-8. https://doi.org/10.1007/s13277-015-4420-0. [PubMed].
- 21. Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS One. 2013; 8: e60021. https://doi.org/10.1371/journal.pone.0060021. [PubMed].
- 22. Indirect immunofluorescence localization of beta-adrenergic receptors and G-proteins in human A431 cells. Biochem J. 1989; 263: 519-32. https://doi.org/10.1042/bj2630519. [PubMed].
- 23. Beta-adrenergic receptors are expressed across diverse cancers. Oncoscience. 2017; 4: 95-105. https://doi.org/10.18632/oncoscience.357. [PubMed].
- 24. . beta-Adrenergic modulation of cancer cell proliferation: available evidence and clinical perspectives. J Cancer Res Clin Oncol. 2017; 143: 275-91. https://doi.org/10.1007/s00432-016-2278-1. [PubMed].
- 25. . Beta Adrenergic Signaling: A Targetable Regulator of Angiosarcoma and Hemangiosarcoma. Veterinary Sciences. 2015; 2: 270. https://doi.org/10.3390/vetsci2030270. [PubMed].
- 26. . Stress, catecholaminergic system and cancer. Stress. 2016; 19: 419-28. https://doi.org/10.1080/10253890.2016.1203415. [PubMed].
- 27. . Safety of Oral Propranolol for the Treatment of Infantile Hemangioma: A Systematic Review. Pediatrics. 2016; 138. https://doi.org/10.1542/peds.2016-0353. [PubMed].
- 28. Pantziarka P, Bouche G, Sukhatme V, Meheus L, Rooman I, Sukhatme VP. Repurposing Drugs in Oncology (ReDO)-Propranolol as an anti-cancer agent. Ecancermedicalscience. 2016; 10: 680. https://doi.org/10.3332/ecancer.2016.680. [PubMed].
- 29. Leaute-Labreze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taieb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008; 358: 2649-51. https://doi.org/10.1056/NEJMc0708819. [PubMed].
- 30. Propranolol treatment of infantile hemangioma endothelial cells: A molecular analysis. Exp Ther Med. 2012; 4: 594-604. https://doi.org/10.3892/etm.2012.654. [PubMed].
- 31. . Targeted therapy with propranolol and metronomic chemotherapy combination: sustained complete response of a relapsing metastatic angiosarcoma. Ecancermedicalscience. 2015; 9: 499. https://doi.org/10.3332/ecancer.2015.499. [PubMed].
- 32. Growth Attenuation of Cutaneous Angiosarcoma With Propranolol-Mediated beta-Blockade. JAMA Dermatol. 2015; 151: 1226-9. https://doi.org/10.1001/jamadermatol.2015.2554. [PubMed].
- 33. . Large nose angiosarcoma treated effectively with oral cyclophosphamide combined with propranolol. J Eur Acad Dermatol Venereol. 2017. https://doi.org/10.1111/jdv.14528. [PubMed].
- 34. . Visceral metastatic angiosarcoma treated effectively with oral cyclophosphamide combined with propranolol. JAAD Case Rep. 2016; 2: 497-9. https://doi.org/10.1016/j.jdcr.2016.10.005. [PubMed].
- 35. Successful Propranolol Treatment of a Kaposiform Hemangioendothelioma Apparently Resistant to Propranolol. Pediatr Blood Cancer. 2016; 63: 1290-2. https://doi.org/10.1002/pbc.25979. [PubMed].
- 36. . Effective Management of Advanced Angiosarcoma by the Synergistic Combination of Propranolol and Vinblastine-based Metronomic Chemotherapy: A Bench to Bedside Study. EBioMedicine. 2016; 6: 87-95. https://doi.org/10.1016/j.ebiom.2016.02.026. [PubMed].
- 37. . Metastatic Primary Angiosarcoma of the Breast: Can We Tame It the Metronomic Way. Indian J Med Paediatr Oncol. 2017; 38: 228-31. [PubMed].
- 38. Wang Z, Li K, Dong K, Xiao X, Zheng S. Variable response to propranolol treatment of kaposiform hemangioendothelioma, tufted angioma, and Kasabach-Merritt phenomenon. Pediatr Blood Cancer. 2014; 61: 1518-9. https://doi.org/10.1002/pbc.24957. [PubMed].
- 39. . alpha2-Adrenergic blockade mimics the enhancing effect of chronic stress on breast cancer progression. Psychoneuroendocrinology. 2015; 51: 262-70. https://doi.org/10.1016/j.psyneuen.2014.10.004. [PubMed].
- 40. . Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: implication in breast cancer treatment. Oncotarget. 2011; 2: 797-809. https://doi.org/10.18632/oncotarget.343. [PubMed].
- 41. . beta-Adrenergic receptors suppress Rap1B prenylation and promote the metastatic phenotype in breast cancer cells. Cancer Biol Ther. 2015; 16: 1364-74. https://doi.org/10.1080/15384047.2015.1070988. [PubMed].
- 42. . beta-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br J Cancer. 2013; 108: 2485-94. https://doi.org/10.1038/bjc.2013.205. [PubMed].
- 43. . Functional involvement of beta3-adrenergic receptors in melanoma growth and vascularization. J Mol Med (Berl). 2013; 91: 1407-19. https://doi.org/10.1007/s00109-013-1073-6. [PubMed].
- 44. Jean Wrobel L, Bod L, Lengagne R, Kato M, Prevost-Blondel A, Le Gal FA. Propranolol induces a favourable shift of anti-tumor immunity in a murine spontaneous model of melanoma. Oncotarget. 2016; 7: 77825-37. https://doi.org/10.18632/oncotarget.12833. [PubMed].
- 45. . Biphasic effects of propranolol on tumour growth in B16F10 melanoma-bearing mice. Br J Pharmacol. 2017; 174: 139-49. https://doi.org/10.1111/bph.13662. [PubMed].
- 46. . Role of host beta1- and beta2-adrenergic receptors in a murine model of B16 melanoma: functional involvement of beta3-adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol. 2015; 388: 1317-31. https://doi.org/10.1007/s00210-015-1165-7. [PubMed].
- 47. Wnorowski A, Sadowska M, Paul RK, Singh NS, Boguszewska-Czubara A, Jimenez L, Abdelmohsen K, Toll L, Jozwiak K, Bernier M, Wainer IW. Activation of beta2-adrenergic receptor by (R,R’)-4’-methoxy-1-naphthylfenoterol inhibits proliferation and motility of melanoma cells. Cell Signal. 2015; 27: 997-1007. https://doi.org/10.1016/j.cellsig.2015.02.012. [PubMed].
- 48. . Propranolol induced G0/G1/S phase arrest and apoptosis in melanoma cells via AKT/MAPK pathway. Oncotarget. 2016; 7: 68314-27. https://doi.org/10.18632/oncotarget.11599. [PubMed].
- 49. . Src activation by beta-adrenoreceptors is a key switch for tumour metastasis. Nat Commun. 2013; 4: 1403. https://doi.org/10.1038/ncomms2413. [PubMed].
- 50. . Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol. 2011; 29: 2635-44. https://doi.org/10.1200/JCO.2010.33.5422. [PubMed].
- 51. . Impact of beta blockers on epithelial ovarian cancer survival. Gynecol Oncol. 2012; 127: 375-8. https://doi.org/10.1016/j.ygyno.2012.07.102. [PubMed].
- 52. . Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol. 2014; 65: 635-41. https://doi.org/10.1016/j.eururo.2013.01.007. [PubMed].
- 53. Grytli HH, Fagerland MW, Fossa SD, Tasken KA, Haheim LL. Use of beta-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapy. Prostate. 2013; 73: 250-60. https://doi.org/10.1002/pros.22564. [PubMed].
- 54. . beta-Blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomarkers Prev. 2011; 20: 2273-9. https://doi.org/10.1158/1055-9965.EPI-11-0249. [PubMed].
- 55. . Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011; 29: 2645-52. https://doi.org/10.1200/JCO.2010.33.4441. [PubMed].
- 56. . Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010; 1: 628-38. https://doi.org/10.18632/oncotarget.197. [PubMed].
- 57. . Non-selective beta-blockers may reduce risk of hepatocellular carcinoma: a meta-analysis of randomized trials. Liver Int. 2015; 35: 2009-16. https://doi.org/10.1111/liv.12782. [PubMed].
- 58. . Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer. 2015; 121: 3444-51. https://doi.org/10.1002/cncr.29392. [PubMed].
- 59. Shaashua L, Shabat-Simon M, Haldar R, Matzner P, Zmora O, Shabtai M, Sharon E, Allweis T, Barshack I, Hayman L, Arevalo J, Ma J, Horowitz M, et al. Perioperative COX-2 and beta-Adrenergic Blockade Improves Metastatic Biomarkers in Breast Cancer Patients in a Phase-II Randomized Trial. Clin Cancer Res. 2017; 23: 4651-61. https://doi.org/10.1158/1078-0432.CCR-17-0152. [PubMed].
- 60. . SVR angiosarcomas can be rejected by CD4 costimulation dependent and CD8 costimulation independent pathways. Mol Med. 2002; 8: 551-8. [PubMed].
- 61. . Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci U S A. 1997; 94: 861-6. https://doi.org/10.1073/pnas.94.3.861. [PubMed].
- 62. . Hemangiosarcoma and its cancer stem cell subpopulation are effectively killed by a toxin targeted through epidermal growth factor and urokinase receptors. Int J Cancer. 2013; 133: 1936-44. https://doi.org/10.1002/ijc.28187. [PubMed].
- 63. Kim JH, Frantz AM, Anderson KL, Graef AJ, Scott MC, Robinson S, Sharkey LC, O’Brien TD, Dickerson EB, Modiano JF. Interleukin-8 promotes canine hemangiosarcoma growth by regulating the tumor microenvironment. Exp Cell Res. 2014; 323: 155-64. https://doi.org/10.1016/j.yexcr.2014.02.020. [PubMed].
- 64. . Interleukin-12 inhibits tumor growth in a novel angiogenesis canine hemangiosarcoma xenograft model. Neoplasia. 2004; 6: 106-16. https://doi.org/10.1593/neo.03334. [PubMed].
- 65. . Establishment of a new murine-phenotypic angiosarcoma cell line (ISOS-1). J Dermatol Sci. 1998; 16: 91-8. https://doi.org/10.1016/s0923-1811(97)00032-7. [PubMed].
- 66. Masuzawa M, Fujimura T, Hamada Y, Fujita Y, Hara H, Nishiyama S, Katsuoka K, Tamauchi H, Sakurai Y. Establishment of a human hemangiosarcoma cell line (ISO-HAS). Int J Cancer. 1999; 81: 305-8. https://doi.org/10.1002/(sici)1097-0215(19990412)81:2<305::aid-ijc22>3.0.co;2-z. [PubMed].
- 67. . Alterations of the p53 and PIK3CA/AKT/mTOR pathways in angiosarcomas: a pattern distinct from other sarcomas with complex genomics. Cancer. 2012; 118: 5878-87. https://doi.org/10.1002/cncr.27614. [PubMed].
- 68. . The RUDY study platform - a novel approach to patient driven research in rare musculoskeletal diseases. Orphanet J Rare Dis. 2016; 11: 150. https://doi.org/10.1186/s13023-016-0528-6. [PubMed].
- 69. . The role of MAPK pathway in bone and soft tissue tumors. Anticancer Res. 2011; 31: 549-53. [PubMed].
- 70. . Social media methods for studying rare diseases. Pediatrics. 2014; 133: e1345-53. https://doi.org/10.1542/peds.2013-2966. [PubMed].
- 71. . Cancer patients on Twitter: a novel patient community on social media. BMC Res Notes. 2012; 5: 699. https://doi.org/10.1186/1756-0500-5-699. [PubMed].
- 72. Svenstrup D, Jorgensen HL, Winther O. Rare disease diagnosis: A review of web search, social media and large-scale data-mining approaches. Rare Dis. 2015; 3: e1083145. https://doi.org/10.1080/21675511.2015.1083145. [PubMed].
- 73. . Using social media in oncology for education and patient engagement. Oncology (Williston Park). 2012; 26: 782, 4-5, 91. [PubMed].
- 74. . Chronic exposure to stress hormones promotes transformation and tumorigenicity of 3T3 mouse fibroblasts. Stress. 2013; 16: 114-21. https://doi.org/10.3109/10253890.2012.686075. [PubMed].
- 75. . Propranolol suppresses angiogenesis in vitro: inhibition of proliferation, migration, and differentiation of endothelial cells. Vascul Pharmacol. 2010; 53: 200-8. https://doi.org/10.1016/j.vph.2010.08.002. [PubMed].
- 76. . Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol Metab. 1999; 10: 359-68. https://doi.org/10.1016/s1043-2760(99)00188-5. [PubMed].
- 77. . Induction of M2 regulatory macrophages through the beta2-adrenergic receptor with protection during endotoxemia and acute lung injury. J Innate Immun. 2014; 6: 607-18. https://doi.org/10.1159/000358524. [PubMed].
- 78. . Propranolol as a modulator of M2b monocytes in severely burned patients. J Leukoc Biol. 2011; 89: 797-803. https://doi.org/10.1189/jlb.1010553. [PubMed].
- 79. . Adrenergic receptor beta2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015; 48: 295-300. https://doi.org/10.5483/BMBRep.2015.48.5.008. [PubMed].
- 80. . Adrenergic modulation of migration, CD11b and CD18 expression, ROS and interleukin-8 production by human polymorphonuclear leukocytes. Inflamm Res. 2015; 64: 127-35. https://doi.org/10.1007/s00011-014-0791-8. [PubMed].
- 81. . Norepinephrine inhibits human natural killer cell activity in vitro. Int J Neurosci. 1991; 58: 127-31. https://doi.org/10.3109/00207459108987189. [PubMed].
- 82. . Impairment of the host’s antibacterial resistance by norepinephrine activated neutrophils. Burns. 2008; 34: 460-6. https://doi.org/10.1016/j.burns.2007.07.004. [PubMed].
- 83. . Natural killer cell anergy to cytokine stimulants in a subgroup of patients with heart failure: relationship to norepinephrine. Neuroimmunomodulation. 1995; 2: 16-24. https://doi.org/10.1159/000096830. [PubMed].
- 84. . beta-adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy. Cancer Res. 2017. https://doi.org/10.1158/0008-5472.CAN-17-0546. [PubMed].
Last Modified: 2018-05-02 16:23:33 EDT
