Oncoscience
KrasG12D upregulates Notch signaling to induce gallbladder tumorigenesis in mice
Wen-Cheng Chung1, Junqing Wang2, Yunyun Zhou3 and Keli Xu1,4
1Cancer Institute, University of Mississippi Medical Center, Jackson, MS, USA
2Department of Surgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
3Department of Data Science, University of Mississippi Medical Center, Jackson, MS, USA
4Department of Neurobiology and Anatomical Sciences, University of Mississippi Medical Center, Jackson, MS, USA
Correspondence to: Keli Xu, email: [email protected]
Keywords: gallbladder tumorigenesis, Kras mutation, Notch signaling, mouse model, adenoma
Received: August 04, 2017
Accepted: September 17, 2017
Published: October 23, 2017
Copyright: Chung et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
Background: Kras mutations and increased Notch activation occur frequently in gallbladder cancer. However, their roles in gallbladder carcinogenesis have not been defined. This study was aimed at determining whether expression of mutant Kras was sufficient to induce gallbladder carcinoma and whether Notch deregulation played a role in this context.
Methods: We determined Cre recombination activity of Pdx1-Cre in the gallbladder using a reporter strain and examined gallbladder tumor development in the KrasLSL-G12D/+;Pdx1-Cre mice. We analyzed expression of Notch pathway genes in the mouse gallbladder by immunohistochemistry, quantitative RT-PCR, and Western blot analysis. We also determined the effect of Jag1 deletion on Kras-induced gallbladder tumor development.
Results: Pdx1-Cre exhibits robust recombination activity in the gallbladder epithelium. KrasLSL-G12D/+;Pdx1-Cre mice form early onset adenoma in the gallbladder and adjacent biliary tract with complete penetrance, albeit short of invasive adenocarcinoma. KrasG12D upregulates expressions of Notch2, Notch3, Notch4, Jag1 and downstream target genes Hes1, Hey1 and Hey2, and deletion of Jag1 partially suppresses KrasG12D-induced adenoma development.
Conclusions: KrasG12D induces gallbladder adenoma and Notch plays a key role in Kras-initiated gallbladder tumorigenesis.
INTRODUCTION
Gallbladder cancer is the most common malignancy of the biliary tract, associated with late diagnosis, unsatisfactory treatment and poor prognosis. There are two key pathways leading to gallbladder carcinogenesis. The first involves gallstones and the resultant cholecystitis. The second involves congenital anomalous pancreatobiliary duct junction (APBDJ). Gallbladder cancers evolved through these two pathways demonstrate differences in demographic distribution, clinical outcome, gender bias and molecular changes. Regarding the molecular changes, KRAS mutations are frequent and early events in tumors associated with APBDJ, but less frequent in carcinomas associated with gallstones [1]. Other molecular changes of gallbladder cancers include somatic mutations of TP53, ErbB signaling pathway genes, and cell cycle regulator CDKN2A [2, 3]. Overexpression of Erbb2 in the basal epithelium of the mouse gallbladder resulted in the development of adenoma and progression to adenocarcinoma characterized by papillary structures, demonstrating a functional role of Erbb2 in gallbladder carcinogenesis [4]. No comprehensive analysis for other molecular changes has been carried out using in vivo model system.Notch signaling controls cell fate determination, cell differentiation and proliferation in a variety of tissues during development as well as in adult homeostasis. Haploinsufficiency for the Notch ligand JAG1 results in an autosomal-dominant, multisystem disorder known as Alagille syndrome, which is characterized by a congenital cholangiopathy of variable severity. Similarly, Jag1 heterozygosity in mice results in impaired intrahepatic bile duct development [5]. Notch2 appears to interact with Jag1 in the pathogenesis of Alagille syndrome [6]. Conversely, high level expression of Notch pathway genes has been found in human gallbladder cancer, and Notch 1 and Notch 3 expression is correlated with severe clinicopathological characteristics and poor prognosis [7–9]. However, the mechanism of Notch deregulation and its role in gallbladder cancer initiation and progression remain unclear.
In the present study, we determined the outcome of expression of the most common form of Kras mutations (KrasG12D) in the mouse gallbladder epithelium. We also determined whether Notch signaling is deregulated by KrasG12D and contributes to the gallbladder tumorigenesis.
RESULTS
Expression of KrasG12D in the gallbladder epithe-lial cells induces adenoma
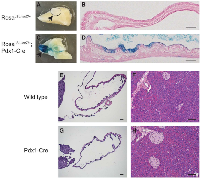
Pdx1 is known to be expressed in endocrine and some duct cells and may identify a more primitive population in the adult pancreas. Interestingly some epithelial cells in adult human gallbladder highly express Pdx1 [10]. We crossed a RosaLSL-lacZ reporter into the Pdx1-Cre mouse and performed X-gal staining in the gallbladder to determine the Cre recombination activity. Robust LacZ activity was observed in a subset of ductal epithelial cells in RosaLSL-lacZ/+;Pdx1-Cre gallbladder, but not in RosaLSL-lacZ+ control (Figure 1A-1D). Therefore, Pdx1-Cre can be used to drive expression of KrasG12D in the gallbladder, in addition to the pancreas as previously reported [11]. Of note, Pdx1-Cre mice undergo normal development of the gallbladder and pancreas (Figure 1E-1H).
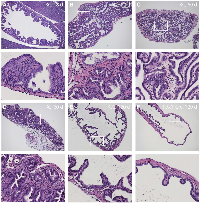
We examined tumor development in the gallbladder of KrasLSL-G12D/+;Pdx1-Cre (KC) mice. As early as 18 days after birth, KC mice showed gallbladder epithelial hyperplasia (Figure 2A). By 2 months of age, all animals examined (10/10) developed gallbladder adenoma prior to the detection of significant lesion in the pancreas. These tumors are composed of papillary structures lined by cuboidal or columnar epithelial cells with moderate atypia (Figure 2B, 2C) or tubular glands (Figure 2D). It is noteworthy that papillary adenomas were prevalent in the gallbladder while tubular glandular lesions occurred more frequently in the adjacent biliary tract. No invasive adenocarcinoma was found in KC mice up to 5 months of age. The histology of the Pdx1-Cre gallbladder is completely normal (Figure 2F).
Expression of KrasG12D causes upregulation of Notch receptors and Jag1 ligand and increased Notch signaling in the gallbladder
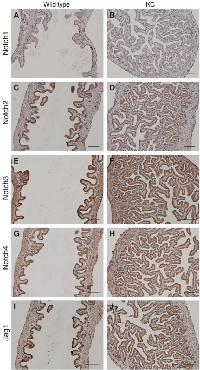
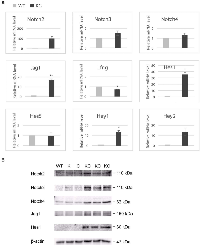
Aberrant expression of Notch receptors has been suggested to play a role in extrahepatic cholangiocarcinoma and gallbladder carcinoma [8]. We performed immunohistochemistry of Notch receptors and Jag1 ligand on the gallbladder sections at 2 months of age. Both wild type and KC mice showed intense staining of Notch2, Notch3, Notch4 and Jag1 in the gallbladder epithelial cells, whereas Notch1 immunoreactivity was very weak or undetectable (Figure 3). We determined the mRNA levels of Notch pathway genes in the gallbladder by quantitative RT-PCR. Transcripts of Notch2 and Jag1 were increased 97- and 17-fold, respectively, while Notch3 and Notch4 mRNA levels were slightly increased in KC as compared to the wild type (Figure 4A). The expression of Lfng, which is known to inhibit Jagged/Serrate-mediated Notch activation [12], was downregulated in KC mice. Transcription of Notch downstream target genes Hes1, Hey1, and Hey2 were all increased drastically in KC mice (Figure 4A). Next, we performed Western blot analysis for Notch pathway components in the gallbladder. KC mice showed consistent increase of the protein levels of Notch2, Notch3, Notch4, Jag1, and Hes1 as compared to control mice including the wild type, KrasLSL-G12D/+ and Pdx1-Cre (Figure 4B). Taken together, Notch signaling is upregulated by the expression of KrasG12D in the gallbladder epithelial cells. Interestingly, 4 out of 8 KrasLSL-G12D/+;Jag1flox/flox;Pdx1-Cre (KJC) mice between 4 and 6 months of age showed near normal histology of the gallbladder, suggesting that deletion of Jag1 partially suppresses KrasG12D-induced adenoma development (Figure 2E).
Expression of Notch pathway genes in the human gallbladder
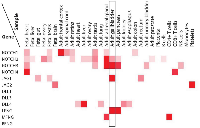
We surveyed protein levels of Notch receptors, ligands, and Fringes in human tissues using published proteomic data (http://www.humanproteomemap.org) [13]. As shown in Figure 5, expressions of NOTCH2, NOTCH3, JAG1 and LFNG are enriched in the adult gallbladder. This is in agreement with our analysis of Notch pathway components in murine gallbladder, suggesting that JAG1-mediated, LFNG-modulated NOTCH2/3 signaling may well play an important role in KRAS-initiated gallbladder carcinogenesis in humans.
DISCUSSION
In a model for multistage pathogenesis of the most common form of gallbladder cancer beginning with gallstones and chronic cholecystitis, Kras mutations are thought to occur at the very late stage [3]. On the other hand, previous study also found higher frequency of Kras mutations in gallbladder adenomas compared with carcinomas, hinting that gallbladder adenomas and carcinomas may arise through distinct molecular pathways [14]. In the present study, the expression of KrasG12D caused papillary adenoma as early as one month of age (data not shown). It is interesting that Kras mutations were found more frequently in patients with papillary adenocarcinoma compared with other types of adenocarcinoma or squamous cell carcinoma [15]. However, early onset adenomas in KC mice do not progress into invasive carcinoma, suggesting additional molecular changes may be required for the development of adenocarcinoma.
Notch signaling pathway plays a central role in the development of biliary system. Hes1-deficient mice exhibit gallbladder agenesis and severe hypoplasia of extrahepatic bile ducts [16]. Double heterozygous mice of Jag1 and Notch2 display jaundice associated with defects in bile duct epithelial cell differentiation and morphogenesis [6]. Here we show that expression of KrasG12D upregulates Notch gene expression, suggesting that Kras may induce gallbladder adenoma through activation of Notch signaling. Hes1 is the most upregulated gene among canonical Notch downstream target genes in the KC gallbladder. Given that loss-of-Hes1 results in gallbladder agenesis, we speculate that Hes1 controls gallbladder epithelial cell proliferation and/or differentiation. Lfng, a negative regulator of Serrate/Jagged-mediated Notch signaling, is highly expressed in the adult human gallbladder. KrasG12D downregulates Lfng expression in the gallbladder, raising the possibility that Lfng normally inhibits Notch activation to prevent gallbladder adenoma formation. It is also likely that Kras-mediated Notch deregulation promotes tumor development in a subset of gallbladder cancer patients, and targeting Notch2/3 and Jag1 could be a therapeutic approach for these patients.
Understanding of gallbladder carcinogenesis is limited in part due to the paucity of animal models for this disease. One model is the overexpression of ErbB2 in the basal layer of biliary tract epithelium under the control of the bovine keratin 5 promoter [4]. Another involves the inactivation of the oxysterol receptor liver X receptor–β in female mice, resulting in preneoplastic lesions of the gallbladder and progression to cancer in old animals [17]. We have shown that Pdx1 promoter is active in the gallbladder epithelium. Thus, forced expression or inactivation of genes in the gallbladder using Pdx1-Cre can be a new approach in developing mouse models for gallbladder cancer.
MATERIALS AND METHODS
Mice
KrasLSL-G12D/+, RosaLSL-lacZ/+ and Pdx1-Cre mouse strains were obtained from the Jackson Laboratory and have been previously described [18–20]. Jag1flox strain was provided by Dr. Radtke and described previously [21]. Mouse experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Histology, immunohistochemistry, X-Gal staining, and western blot
Formalin-fixed paraffin-embedded gallbladder tissues were processed for histology and immunohistochemistry by standard procedures. Primary antibodies used for immunostaining were: Notch1 (Cell Signaling, No. 3608, 1:100), Notch2 (DSHB, University of Iowa, C651.6DbHN, 1:200), Notch3 (ProteinTech, 55114-1-AP, 1:100), Notch4 (Millipore, 09-089, 1:100), and Jagged1 (Santa Cruz, sc-6011, 1:100). X-Gal staining was performed as previously described [22]. For Western blot analysis, gallbladder tissues were lysed in RIPA buffer (Boston BioProducts) supplemented with protease inhibitor (Roche), and processed according to standard methodology. Antibodies for probing specified proteins are as follows: Notch1, Notch2, Notch3, Notch4 and Jagged1 are the same as above (all with 1:1000 dilution), Hes1 (Millipore, AB5702, 1:500), and β-Actin (Santa Cruz, sc-81178, 1:1000).
Quantitative RT-PCR
Total RNA was extracted from the gallbladder using RNeasy Mini kit (Qiagen) and reverse-transcribed using iScript cDNA synthesis kit (Bio-rad). PCR was performed using QuantiTect SYBR Green PCR Kits (Qiagen) with BioRad CFX96 qPCR System. Results were normalized with the expression level of Gapdh and presented as fold change of the control. The experiment was performed in triplicate and presented as mean ± standard error. Unpaired two-tailed t-test was performed for comparison between the mutant and wild type mice. Primer sequences for Jag1, Lfng, Hes1, Hey1 and Hey2 have previously been reported [11, 23]. Other primer sequences are as follows: Notch2, 5’-AACTGTCAGACCCTGGTGAAC-3’ (forward), 5’-CGACAAGTGTAGCCTCCAATC-3’ (reverse); Notch3, 5’-TGCCAGAGTTCAGTGGTGG-3’ (forward), 5’-CACAGGCAAATCGGCCATC-3’ (reverse); Notch4, 5’-AGGGAGGCTCTGTGAGGTGG-3’ (forward), 5’-ATCCAGGAAGGCAGAGGCAC-3’ (reverse).
Abbreviations
APBDJ, anomalous pancreatobiliary duct junction, KC, KrasLSL-G12D/+;Pdx1-Cre, KJC, KrasLSL-G12D/+;Jag1flox/flox;Pdx1-Cre.
Author contributions
KX and WCC contributed to the conception and design of the study, the acquisition, analysis and interpretation of the data, writing and reviewing of the manuscript. YZ and JW contributed to the analysis and interpretation of data and reviewing of the manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Freddy Radtke for providing the Jag1 mouse strain.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
FUNDING
This work was supported by the Intramural Research Support Program of the University of Mississippi Medical Center.
- 1. K-ras point mutations in cancerous and noncancerous biliary epithelium in patients with pancreaticobiliary maljunction. Cancer. 1996; 77:1752-1757. https://doi.org/10.1002/(SICI)1097-0142(19960415)77:8%3C1752::AID-CNCR51%3E3.0.CO;2-V. [PubMed].
- 2. Li M, Zhang Z, Li X, Ye J, Wu X, Tan Z, Liu C, Shen B, Wang XA, Wu W, Zhou D, Zhang D, Wang T, et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat Genet. 2014; 46:872-876. https://doi.org/10.1038/ng.3030. [PubMed].
- 3. Gallbladder cancer: lessons from a rare tumour. Nat Rev Cancer. 2004; 4:695-706. https://doi.org/10.1038/nrc1429. [PubMed].
- 4. Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res. 2001; 61:6971-6976. [PubMed].
- 5. Jagged1 heterozygosity in mice results in a congenital cholangiopathy which is reversed by concomitant deletion of one copy of Poglut1 (Rumi). Hepatology. 2016; 63:550-565. https://doi.org/10.1002/hep.28024. [PubMed].
- 6. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development. 2002; 129:1075-1082. [PubMed].
- 7. Mazur PK, Riener MO, Jochum W, Kristiansen G, Weber A, Schmid RM, Siveke JT. Expression and clinicopathological significance of notch signaling and cell-fate genes in biliary tract cancer. Am J Gastroenterol. 2012; 107:126-135. https://doi.org/10.1038/ajg.2011.305. [PubMed].
- 8. Clinicopathological significance of altered Notch signaling in extrahepatic cholangiocarcinoma and gallbladder carcinoma. World J Gastroenterol. 2011; 17:4023-4030. https://doi.org/10.3748/wjg.v17.i35.4023. [PubMed].
- 9. The Expression of Notch 1 and Notch 3 in Gallbladder Cancer and Their Clinicopathological Significance. Pathol Oncol Res. 2016; 22:483-492. https://doi.org/10.1007/s12253-015-0019-4. [PubMed].
- 10. Novel surface markers directed against adult human gallbladder. Stem Cell Res. 2015; 15:172-181. https://doi.org/10.1016/j.scr.2015.06.004. [PubMed].
- 11. Lunatic Fringe is a potent tumor suppressor in Kras-initiated pancreatic cancer. Oncogene. 2016; 35:2485-2495. https://doi.org/10.1038/onc.2015.306. [PubMed].
- 12. Glycosylation regulates Notch signalling. Nature reviews Molecular cell biology. 2003; 4:786-797. https://doi.org/10.1038/nrm1228. [PubMed].
- 13. A draft map of the human proteome. Nature. 2014; 509:575-581. https://doi.org/10.1038/nature13302. [PubMed].
- 14. Mutations in the RAS/RAF/MAP kinase pathway commonly occur in gallbladder adenomas but are uncommon in gallbladder adenocarcinomas. Appl Immunohistochem Mol Morphol. 2011; 19:133-140. https://doi.org/10.1097/PAI.0b013e3181f09179. [PubMed].
- 15. Gallbladder cancers: associated conditions, histological types, prognosis, and prevention. Eur J Gastroenterol Hepatol. 2014; 26:562-569. https://doi.org/10.1097/MEG.0000000000000074. [PubMed].
- 16. Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004; 36:83-87. https://doi.org/10.1038/ng1273. [PubMed].
- 17. Estrogen-dependent gallbladder carcinogenesis in LXRbeta-/- female mice. Proc Natl Acad Sci U S A. 2010; 107:14763-14768. https://doi.org/10.1073/pnas.1009483107. [PubMed].
- 18. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003; 4:437-450. https://doi.org/10.1016/s1535-6108(03)00309-x. [PubMed].
- 19. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes & development. 2001; 15:3243-3248. https://doi.org/10.1101/gad.943001. [PubMed].
- 20. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999; 21:70-71. https://doi.org/10.1038/5007. [PubMed].
- 21. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005; 105:2340-2342. https://doi.org/10.1182/blood-2004-08-3207. [PubMed].
- 22. Lunatic fringe deficiency cooperates with the Met/Caveolin gene amplicon to induce basal-like breast cancer. Cancer cell. 2012; 21:626-641. https://doi.org/10.1016/j.ccr.2012.03.041. [PubMed].
- 23. Jagged1 is the major regulator of notch-dependent cell fate in proximal airways. Dev Dyn. 2013. https://doi.org/10.1002/dvdy.23965. [PubMed].
Last Modified: 2017-10-30 22:29:28 EDT

