Oncoscience
Abstract | PDF | Full Text | Supplementary Materials | How to Cite
https://doi.org/10.18632/oncoscience.23
HER2 and HER3 cooperatively regulate cancer cell growth and determine sensitivity to the novel investigational EGFR/HER2 kinase inhibitor TAK-285
Shinji Takagi1, Hiroshi Banno1, Akira Hayashi1, Toshiya Tamura1, Tomoyasu Ishikawa1, Yoshikazu Ohta1
1 Oncology Drug Discovery Unit, Pharmaceutical Research Division, Takeda Pharmaceutical Company Ltd., Fujisawa, Kanagawa, Japan
Correspondence to: Shinji Takagi, email: shinji.takagi@takeda.com
Keywords: HER2, HER3, EGFR, kinase inhibitor, drug sensitivity, TAK-285
Received: February 2, 2014
Accepted: March 24, 2014
Published: March 24, 2014
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT:
The human epidermal growth factor receptor (HER) family plays a major role in cancer cell proliferation. Overexpression of these receptors occurs in various cancers, including breast cancer, and correlates with shorter time to relapse and lower overall survival. We recently reported that TAK-285, an orally bioavailable small molecule inhibitor of HER kinases, is not a p-glycoprotein substrate and penetrates the blood-brain barrier, suggesting favorable activity for the treatment of brain metastases. To identify the determinants of sensitivity to TAK-285, we examined the relationship between the IC50 values of TAK-285 for cell growth inhibition and the expression of candidate genes that are involved in the HER family signaling pathway and trastuzumab resistance in a panel of human breast cancer cell lines, other types of cancer cells, and non-transformed cells in vitro. These analyses showed an inverse correlation between sensitivity to TAK-285 (IC50 values) and HER2 or HER3 expression. HER3 was highly phosphorylated in TAK-285-sensitive cells, where TAK-285 treatment reduced HER3 phosphorylation level. Because HER3 does not possess kinase activity and a selective inhibitor of HER2 but not of an epidermal growth factor receptor reduced the phospho-HER3 level, HER3 was suggested to be trans-phosphorylated by HER2. HER3 knockdown using small interfering RNA (siRNA) inhibited cancer cell growth in TAK-285-sensitive cells but not in TAK-285-insensitive cells. These results suggest that HER2 and HER3 mainly regulate cancer cell growth in TAK-285-sensitive cells and that phospho-HER3 could be used as a potential molecular marker to select patients most likely to respond to TAK-285.
INTRODUCTION
HER (ErbB) family consists of epidermal growth factor receptor (EGFR; HER1, ErbB1), HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4). These receptors possess intrinsic tyrosine kinase activity within the intracellular domain except HER3. After binding of ligands such as epidermal growth factor (EGF) or heregulin to receptors, biological effects are exerted through homo- or heterodimerization [1, 2].
EGFR was identified as a receptor for EGF with high similarity to the v-erbB oncogene of avian erythroblastic leukemia virus [3]. EGFR is overexpressed in various tumors and correlates with patients’ poor prognosis [4]. HER2 was originally identified as an activated protein in rat neuroblastoma, and it possesses homology to EGFR [5, 6]. Moreover, isolated cDNA has transforming activity [7]. HER2 is overexpressed in approximately 25% of breast cancer patients and is a poor prognostic marker [8]. HER2 is also overexpressed in various cancers such as ovarian, lung, and prostate cancers [9]. In clinical settings, EGFR kinase inhibitors such as gefitinib and erlotinib are used for advanced non-small cell lung cancer. The anti-HER2 antibody trastuzumab is used for the treatment of HER2-overexpressing breast cancer patients and the EGFR/HER2 kinase inhibitor lapatinib is used for the treatment of HER2-overexpressing metastatic breast cancer patients who have progressed on trastuzumab therapy. Although HER3 does not possess intrinsic kinase activity, HER3 can form heterodimers upon ligand binding and activate the phosphatidylinositol 3-kinase (PI3K) pathway. HER3 is also believed to be an attractive drug target for cancer therapy [10].
Molecular targeted drugs have been tested in clinics and to identify patients likely to respond to the drugs is of importance for receiving clinical benefit from them. For example, Abl kinase inhibitors such as imatinib and nilotinib impede the proliferation of cells expressing the BCR-ABL fusion protein in vitro and have been proven to be beneficial on the population in clinical settings [11, 12]. Moreover, the clinical benefits of anti-HER2 therapies have been shown in patients with HER2 overexpression or HER2 amplification, confirmed by immunohistochemistry and fluorescence in situ hybridization (FISH), respectively.
We recently reported that the novel investigational EGFR/HER2 kinase inhibitor TAK-285, which has anti-tumor activity and penetrates the rat blood-brain barrier, might be used for the treatment of HER2-overexpressing metastatic breast cancers [13-17]. We here searched for the determinants of sensitivity to TAK-285 and revealed high HER3 phosphorylation in TAK-285-sensitive cells. Subsequent pharmacological and siRNA experiments demonstrated that HER3 is mainly phosphorylated by HER2 and not by EGFR and that it plays an important role in the proliferation of TAK-285-sensitive cells. Therefore, phospho-HER3 could be used as a potential biomarker to select patients likely to respond to TAK-285.
RESULTS
HER3 or HER2 gene expression is inversely correlated to IC50 values of TAK-285
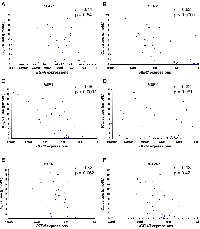
To identify the determinants of sensitivity to TAK-285, we examined the relationship between the IC50 values of TAK-285 for cell growth inhibition and EGFR, HER2, HER3, HER4, phosphatase and tensin homolog (PTEN), and IGF-1R gene expression in a panel of human breast cancer cell lines, other types of cancer cells, and non-transformed cells in vitro. These genes were selected because they reportedly regulate the HER family signaling pathway and trastuzumab resistance [18, 19]. TAK-285 dose-dependently inhibited the proliferation of all cell lines tested. The IC50 values of TAK-285 were determined in 35 cell lines and ranged widely (0.011~17 μmol/L), as described in Supplementary Table 1. Pearson’s coefficient (r) indicated an inverse correlation between the IC50 values of TAK-285 and HER2 or HER3 gene expression (Figure 1).
HER3 is highly phosphorylated in TAK-285-sensitive breast cancer cell lines
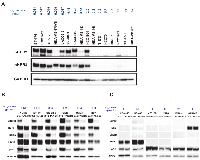
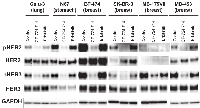
Immunoblot analyses showed coexpression of HER2 and HER3 in TAK-285-sensitive cell lines (Supplementary Figure 1A). We assumed that HER2 and HER3 cooperatively regulate the proliferation of TAK-285-sensitive cancer cells. We determined the relationship between the IC50 values of TAK-285 and phospho-HER3 expression levels in 16 breast cancer cell lines. Phospho-HER3 was detected in TAK-285-sensitive cells (Figure 2A). This result shows that HER3 is activated in these cells. HER2 was not highly expressed in MDA-MB-175VII cells, but HER3 was highly phosphorylated (Figures 2A and 3). Wilson et al. recently showed that HER3 is activated by its ligand neuregulin-1 (NRG-1) in MDA-MB-175VII cells [20]. To investigate the effect of TAK-285 on the phospho-HER3 level, TAK-285-sensitive cell lines were treated with TAK-285 and immunoblot analysis was performed. TAK-285 treatment reduced the level of both phopho-HER2 and phospho-HER3 in sensitive cell lines (Figure 2B). HER3 possesses at least six PI3K binding sites and plays an important role in activating the Akt signaling pathway [21, 22]. Therefore, we examined phospho-Akt levels in cell lysates and found that the levels were clearly decreased after TAK-285 treatment of TAK-285-sensitive cell lines (Figure 2B). In A431 cells that overexpress wild-type EGFR, TAK-285 treatment led to reduced phospho-HER3 and phospho-Akt levels, despite the absence of phospho-HER2 or HER2 (Supplementary Figure 1B). In contrast, phospho-HER2 and phospho-HER3 were not detected, and phospho-Akt was not decreased in TAK-285-insensitive cell lines after the treatment of TAK-285 (Figure 2C). These data indicate that phospho-HER3 could be a potential predictive marker for TAK-285 sensitivity.
It is known that HER3 does not possess intrinsic kinase activity and is trans-phosphorylated by EGFR or HER2 [23, 24]. To determine which members of the HER family phosphorylate HER3, we treated TAK-285-sensitive cells with the selective EGFR and HER2 kinase inhibitors erlotinib and CP-724,714, respectively [25, 26] and performed immunoblot analyses of phospho-HER2 and phospho-HER3. CP-724,714, but not erlotinib, reduced phospho-HER2 and phospho-HER3 in TAK-285-sensitive cells (Figure 3). In addition, erlotinib showed weaker anti-proliferative effects than CP-724,714 in the TAK-285-sensitive cell line HCC1419 (Supplementary Figure 1C). These observations suggest that HER3 is phosphorylated by HER2 in most TAK-285-sensitive cells and that HER2:HER3 heterodimer plays important roles in the proliferation of these cells.
HER3 positively regulates proliferation of TAK-285-sensitive cells
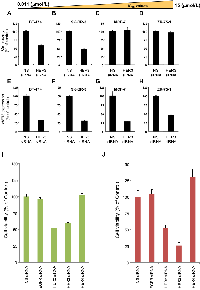
To investigate the involvement of HER3 in cancer cell proliferation, we examined the effects of HER3 knockdown in TAK-285-sensitive and -insensitive cells. The cells were transfected with nonsilencing siRNA (NS siRNA) or HER3 siRNA. Cell growth inhibition assays (Figure 4, Upper panel, A-D) were performed, and HER3 gene expression was quantified relative to GAPDH gene expression by quantitative PCR (Figure 4, Lower panel, E-H). HER3 protein knockdown was confirmed by immunoblot analysis (Supplementary Figure 2A). BT-474 and SK-BR-3 cells were used as TAK-285-sensitive cell lines. MCF-7 and ZR-75-1 cells were used as TAK-285-insensitive cell lines. Although the level of HER3 mRNA expression was reduced after HER3 siRNA transfection in all cell lines tested, cell proliferation was inhibited only in TAK-285-sensitive cell lines, suggesting that HER3 positively regulates the growth of TAK-285-sensitive cells. The HER family consists of EGFR, HER2, HER3, and HER4. Therefore, we examined the effects of knockdown of individual HER family members on the proliferation of TAK-285-sensitive cells. The BT-474 and SK-BR-3 cells were transfected with NS or HER-specific siRNA and growth inhibition assays were performed. Specificity of each siRNA was confirmed by quantitative PCR analysis (Supplementary Figure 2B). In these experiments, siRNA-mediated HER2 or HER3 knockdown, but not EGFR or HER4, inhibited the proliferation of TAK-285-sensitive cells (Figure 4I and J), indicating upregulation of HER2:HER3 signaling in TAK-285-sensitive cell lines. These data further indicate that phospho-HER3 could be used as a potential molecular marker for selecting patients likely to respond to TAK-285.
DISCUSSION
Selection of patients likely to respond to molecular targeted therapeutics is of increasing clinical importance. For example, approximately 4% of non-small-cell lung cancers express the EML4-ALK fusion protein, providing a marker for responsiveness to the ALK kinase inhibitor crizotinib [27, 28]. Indeed, predictions of responses to molecular targeted drugs would increase the benefits for responsive individuals and the opportunities to protect them from unnecessary adverse effects.
The present data show that HER2 and HER3 are coexpressed and positively regulate the proliferation of TAK-285-sensitive cell lines. Moreover, HER3 was highly phosphorylated in TAK-285-sensitive cells and TAK-285 treatment reduced the phospho-HER3 level in these cells. Thus, we propose that phospho-HER3 could be used as a molecular biomarker to select patients likely to respond to TAK-285. Lee-Hoeflich et al. reported that the phospho-HER3 level is highly upregulated in HER2-amplified breast cancer patient tissues [29]. We speculated that HER2:HER3 heterodimer plays an important role in the maintenance of cell proliferation in not only cultured cell lines but also clinical settings. Although other HER family members might be involved in cell proliferation, the present pharmacological approach using a selective HER2 or EGFR inhibitor and RNA interference-mediated knockdown revealed that the HER2:HER3 heterodimer is a predominant regulator of the proliferation of TAK-285-sensitive cells. Engelman et al. and Buck et al. reported high HER3 expression and phosphorylation in EGFR kinase inhibitor-sensitive cancer cell lines [30, 31]. In agreement with previous study results, the present data suggest that HER3 is a common key regulator of the proliferation of EGFR- and HER2-dependent cancer cells.
We showed that MDA-MB-175VII cells with low HER2 expression are sensitive to TAK-285. Wilson et al. recently reported that this cell line overexpresses NRG-1 and demonstrated that a NRG-1-mediated autocrine loop activates HER3 via HER2 kinase [20]. Although we could not detect phospho-HER2 in MDA-MB-175VII cells by immunoblot analysis (Figure 2A), TAK-285 and CP-724,714 treatments reduced the phospho-HER3 level (Figure 3 and data not shown). These data suggest that weak HER2 activity might trans-phosphorylate HER3 and may be indispensable for the maintenance of the proliferation of MDA-MB-175VII cells. SK-OV-3 cells harbor both HER2 amplification and PIK3CA (H1047R) mutation. In these cells, TAK-285 did not reduce the phospho-Akt level (Figure 2C), suggesting that the SK-OV-3 cells are resistant to TAK-285 because of PIK3A mutation-mediated activation of the Akt pathway or the lack of HER3 expression (Figure 2C). In this cell line, the Akt signaling pathway could be activated by PIK3CA mutation irrespective of HER2 amplification. Loss of PTEN or PIK3CA mutation confers resistance to trastuzumab and lapatinib [32-34], although conflicting reports have been reported [35, 36].
Control of the HER3-Akt signaling pathway may be an important strategy for avoiding acquired resistance, as HER3 and Akt were reactivated after prolonged exposure to gefitinib [37]. HER3 and Akt were completely inhibited 6 h after TAK-285 treatment, whereas they were slightly reactivated 48 h after treatment (Supplementary Figure 3). In addition, TAK-285 treatment increased the HER3 protein level, similar to gefitinib treatment ([38] and Supplementary Figure 3). Further TAK-285 exposure may recover Akt activity via kinase-inactive HER3. Therefore, the combination of the Akt or PI3K kinase inhibitor with TAK-285 is a reasonable approach to prevent or delay drug resistance.
In this study, we analyzed the expression and phosphorylation of genes and proteins involved in HER signaling and identified a potential molecular marker for TAK-285 sensitivity. The present data indicate that patients likely to respond to TAK-285 might be identified prospectively on the basis of phospho-HER3 expression. Moreover, tumors with high phospho-HER3 levels appear to include TAK-285-sensitive subpopulations with and without HER2-amplification, such as MDA-MB-175VII cells with low HER2 expression.
MATERIALS AND METHODS
Cells and reagents
A2780, A375, A431, AU565, BT-474, BT-483, Calu-3, Cell System-Fb, H2228, HCC1419, HCC1937, HCC1954, HCC70, HCT116, HCC4006, HT-29, KYSE-30, K562, MCF-7, MCF-10A, MDA-MB-175VII, MDA-MB-231, MDA-MB-435s, MDA-MB-468, MDA-MB-361, MDA-MB-453 MES-SA, MES-SA/Dx-5, MRC-5, NCI-N87, NCI-H1781, OE19, OE21, OE33, OV-90, PC-3, SK-BR-3, SK-OV-3, T-47D, UACC812, UACC893, and ZR-75-1 cells were purchased from commercial sources and were maintained in media as prescribed by the suppliers. TAK-285 was synthesized at Takeda Pharmaceutical Company, Ltd. CP-724,714 was synthesized at Takeda Pharmaceutical Company, Ltd., according to published procedures [26, 39]. Erlotinib hydrochloride was extracted from Tarceva (Roche) at Takeda Pharmaceutical Company, Ltd.
Growth inhibition assay
Cells were seeded into 96-well plates and treated with TAK-285 on the following day. Relative cell numbers were estimated by the sulforhodamine B staining method or the CellTiter-Glo assay (Promega). IC50 values for cell growth inhibition were calculated using SAS software (version 5.0).
Quantitative PCR
Total RNA was extracted from each cell line using the RNeasy mini kit (Qiagen). Gene expression assays for EGFR, HER2, HER3, HER4, and glyceraldhyde-3-phosphate dehydrogenase (GAPDH) were purchased from Applied Biosystems. For data analysis, the Ct value was normalized to the Ct of GAPDH for each sample in order to obtain ∆Ct and then the normalized ∆Ct was calibrated to control samples to calculate ∆∆Ct values.
Immunoblot analysis
Cells were seeded into 24-well plates and treated with compounds for 2 h on the following day. Growth medium was removed and cells were lysed. Protein was resolved by SDS-PAGE and transferred to polyvinylidene fluoride membranes. The antibodies used in this study were specific for EGFR (#2232), HER2 (#2242), pHER2 (Tyr1248; #2247), pHER3 (Tyr1289; #4791), pAkt (Ser473; #9271, Cell Signaling Technology), HER3 (C-17, Santa Cruz Biotechnology), and GAPDH (ab9484-100, Abcam).
Small interfering RNA (siRNA) transfection
Non-silencing (NS) control (Cat. No. 1022076), HER2 (Cat. No. SI02223571), and HER4 (Cat. No. SI00074193) siRNAs were purchased from Qiagen. EGFR siRNA (Cat. No. M-003114-01-05) and HER3 siRNA (Cat. No. sc-35327) were purchased from Dharmacon and Santa Cruz Biotechnology, respectively. Cells were transfected with 5 nmol/L siRNAs using LipofectAMINE 2000 (Invitrogen). Cell growth inhibition assays were performed, as described above, 3 or 5 days after transfection. Total RNA was extracted 2 days after transfection.
FUNDING
This study is supported by Takeda Pharmaceutical Company, LTD.
ACKNOWLEDGEMENTS
We thank members of the Ohta group and the TAK-285 team for helpful discussions and Dr. Osamu Nakanishi and Dr. Shuichi Furuya for their support during the course of this study.
AUTHORSHIP
S.T. designed the study, performed experiments, analyzed data, and drafted the manuscript. H.B. synthesized CP-724,714. T.I. extracted erlotinib from Tarceva. A.H. and T.T. supported experiments. Y.O. critically revised the manuscript. S.T. and Y.O. discussed the results and commented on the manuscript.
CONFLICT OF INTEREST
All authors are employees of Takeda Pharmaceutical Company, Ltd.
- 1. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001; 2: 127-137. https://doi.org/10.1038/35052073. [PubMed].
- 2. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006; 7: 505-516. https://doi.org/10.1038/nrm1962. [PubMed].
- 3. Molecular cloning and characterization of the chicken DNA locus related to the oncogene erbB of avian erythroblastosis virus. Embo J. 1982; 1: 237-242. [PubMed].
- 4. EGFR and cancer prognosis. Eur J Cancer. 2001; 37 Suppl 4: S9-15. https://doi.org/10.1016/s0959-8049(01)00231-3. [PubMed].
- 5. A v-erbB-related protooncogene, c-erbB-2, is distinct from the c-erbB-1/epidermal growth factor-receptor gene and is amplified in a human salivary gland adenocarcinoma. Proc Natl Acad Sci U S A. 1985; 82: 6497-6501. https://doi.org/10.1073/pnas.82.19.6497. [PubMed].
- 6. The c-erbB-2 gene encodes a receptor-like protein with tyrosine kinase activity. Cold Spring Harb Symp Quant Biol. 1986; 51 Pt 2: 977-982. https://doi.org/10.1101/sqb.1986.051.01.111. [PubMed].
- 7. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986; 319: 226-230. https://doi.org/10.1038/319226a0. [PubMed].
- 8. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987; 235: 177-182. https://doi.org/10.1126/science.3798106. [PubMed].
- 9. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989; 244: 707-712. https://doi.org/10.1126/science.2470152. [PubMed].
- 10. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009; 9: 463-475. https://doi.org/10.1038/nrc2656. [PubMed].
- 11. The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of BCR-ABL-positive cells. Blood. 1997; 90: 3691-3698. [PubMed].
- 12. Imatinib and beyond--exploring the full potential of targeted therapy for CML. Nat Rev Clin Oncol. 2009; 6: 535-543. https://doi.org/10.1038/nrclinonc.2009.112. [PubMed].
- 13. Structural analysis of the mechanism of inhibition and allosteric activation of the kinase domain of HER2 protein. J Biol Chem. 2011; 286: 18756-18765. https://doi.org/10.1074/jbc.M110.206193. [PubMed].
- 14. Design and synthesis of novel human epidermal growth factor receptor 2 (HER2)/epidermal growth factor receptor (EGFR) dual inhibitors bearing a pyrrolo[3,2-d]pyrimidine scaffold. J Med Chem. 2011; 54: 8030-8050.
- 15. Doi T, Takiuchi H, Ohtsu A, Fuse N, Goto M, Yoshida M, Dote N, Kuze Y, Jinno F, Fujimoto M, Takubo T, Nakayama N, Tsutsumi R. Phase I first-in-human study of TAK-285, a novel investigational dual HER2/EGFR inhibitor, in cancer patients. Br J Cancer. 2012; 106: 666-672. https://doi.org/10.1038/bjc.2011.590. [PubMed].
- 16. Verification of brain penetration of the unbound fraction of a novel HER2/EGFR dual kinase inhibitor (TAK-285) by microdialysis in rats. Brain Res Bull. 2012; 87: 413-419. https://doi.org/10.1016/j.brainresbull.2012.01.002. [PubMed].
- 17. Nakayama A, Takagi S, Yusa T, Yaguchi M, Hayashi A, Tamura T, Kawakita Y, Ishikawa T, Ohta Y. Antitumor Activity of TAK-285, an Investigational, Non-Pgp Substrate HER2/EGFR Kinase Inhibitor, in Cultured Tumor Cells, Mouse and Rat Xenograft Tumors, and in an HER2-Positive Brain Metastasis Model. J Cancer. 2013; 4: 557-565. https://doi.org/10.7150/jca.6689. [PubMed].
- 18. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004; 6: 117-127. https://doi.org/10.1016/j.ccr.2004.06.022. [PubMed].
- 19. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005; 65: 11118-11128. https://doi.org/10.1158/0008-5472.CAN-04-3841. [PubMed].
- 20. Neuregulin-1-mediated autocrine signaling underlies sensitivity to HER2 kinase inhibitors in a subset of human cancers. Cancer Cell. 2011; 20: 158-172. https://doi.org/10.1016/j.ccr.2011.07.011. [PubMed].
- 21. Cloning of the rat ErbB3 cDNA and characterization of the recombinant protein. Gene. 1995; 165: 279-284. https://doi.org/10.1016/0378-1119(95)00436-a. [PubMed].
- 22. Kim HH, Sierke SL, Koland JG. Epidermal growth factor-dependent association of phosphatidylinositol 3-kinase with the erbB3 gene product. J Biol Chem. 1994; 269: 24747-24755. [PubMed].
- 23. The erbB3 gene product is a receptor for heregulin. J Biol Chem. 1994; 269: 14303-14306. [PubMed].
- 24. Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci U S A. 1994; 91: 8132-8136. https://doi.org/10.1073/pnas.91.17.8132. [PubMed].
- 25. Pollack VA, Savage DM, Baker DA, Tsaparikos KE, Sloan DE, Moyer JD, Barbacci EG, Pustilnik LR, Smolarek TA, Davis JA, Vaidya MP, Arnold LD, Doty JL, Iwata KK, Morin MJ. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J Pharmacol Exp Ther. 1999; 291: 739-748. [PubMed].
- 26. Discovery and pharmacologic characterization of CP-724,714, a selective ErbB2 tyrosine kinase inhibitor. Cancer Res. 2007; 67: 9887-9893. https://doi.org/10.1158/0008-5472.CAN-06-3559. [PubMed].
- 27. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007; 448: 561-566. https://doi.org/10.1038/nature05945. [PubMed].
- 28. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010; 363: 1693-1703. https://doi.org/10.1056/NEJMoa1006448. [PubMed].
- 29. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008; 68: 5878-5887. https://doi.org/10.1158/0008-5472.CAN-08-0380. [PubMed].
- 30. Engelman JA, Janne PA, Mermel C, Pearlberg J, Mukohara T, Fleet C, Cichowski K, Johnson BE, Cantley LC. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc Natl Acad Sci U S A. 2005; 102: 3788-3793. https://doi.org/10.1073/pnas.0409773102. [PubMed].
- 31. Inactivation of Akt by the epidermal growth factor receptor inhibitor erlotinib is mediated by HER-3 in pancreatic and colorectal tumor cell lines and contributes to erlotinib sensitivity. Mol Cancer Ther. 2006; 5: 2051-2059. https://doi.org/10.1158/1535-7163.MCT-06-0007. [PubMed].
- 32. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007; 12: 395-402. https://doi.org/10.1016/j.ccr.2007.08.030. [PubMed].
- 33. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008; 68: 9221-9230. https://doi.org/10.1158/0008-5472.CAN-08-1740. [PubMed].
- 34. PI3K pathway activation results in low efficacy of both trastuzumab and lapatinib. BMC Cancer. 2011; 11: 248. https://doi.org/10.1186/1471-2407-11-248. [PubMed].
- 35. Xia W, Husain I, Liu L, Bacus S, Saini S, Spohn J, Pry K, Westlund R, Stein SH, Spector NL. Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res. 2007; 67: 1170-1175. https://doi.org/10.1158/0008-5472.CAN-06-2101. [PubMed].
- 36. Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J Clin Oncol. 2011; 29: 166-173.
- 37. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007; 445: 437-441. https://doi.org/10.1038/nature05474. [PubMed].
- 38. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A. 2011; 108: 5021-5026. https://doi.org/10.1073/pnas.1016140108. [PubMed].
- 39. a. Preparation of substituted 4-quinazolinamines for the treatment of abnormal cell growth. PCT International 2001; WO0198277.
Last Modified: 2016-06-11 05:50:47 EDT
