Oncoscience
mTOR pathway activation in multiple myeloma cell lines and primary tumour cells: pomalidomide enhances cytoplasmic-nuclear shuttling of mTOR protein
Tommasina Guglielmelli1, Emilia Giugliano1, Vanessa Brunetto1, Ida Rapa2, Susanna Cappia2, Jessica Giorcelli2, Sokol Rrodhe1, Mauro Papotti2, Giuseppe Saglio1
1 Department of Clinical and Biological Sciences, University of Turin and S Luigi Hospital, Orbassano, Turin, Italy
2 Department of Oncology, University of Turin and S Luigi Hospital, Orbassano, Turin, Italy
Correspondence to: Tommasina Guglielmelli, email: [email protected]
Keywords: multiple myeloma, pomalidomide, mTOR pathway, nuclear, AKT
Received: January 13, 2015
Accepted: March 16, 2015
Published: April 6, 2015
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
mTOR is a protein kinase that plays a central role in regulating critical cellular processes. We evaluated the activation and cellular localization of the mTOR pathway in multiple myeloma (MM) and analyzed the role of pomalidomide in regulating mTOR. By immunohistochemistry cytoplasmic p-mTOR stained positive in 57 out 101 (57.6%) cases with a nuclear p-mTOR localization in 14 out 101 samples (13.8%). In the 70 MM samples analyzed for the entire pathway, p-mTOR expression significantly correlated with p-AKT, p-P70S6K, and p-4E-BP1 suggesting that the AKT/mTOR pathway is activated in a subset of MM patients. Immunofluorescence assays demonstrated that mTOR protein is distributed throughout the cytoplasm and the nucleus at baseline in MM cell lines and in plasma cells of 13 MM patients and that pomalidomide facilitated the shift of the mTOR protein in the nucleus. By western blotting, treatment with pomalidomide increased nuclear mTOR and p-mTOR expression levels in the nucleus with a concomitant decrease of the cytoplasmic fractions while does not seem to affect significantly AKT phosphorylation status. In MM cells the anti-myeloma activity of pomalidomide may be mediated by the regulation of the mTOR pathway.
INTRODUCTION
Multiple myeloma (MM) is a malignant plasma cell disorder and remains an incurable disease. The availability of agents such as the proteasome inhibitor bortezomib and the immunomodulatory agents lenalidomide and pomalidomide has considerably increased the treatment options of MM patients [1, 2]. N ew drugs and new combinations are needed to further improve MM patients’ survival. Preclinical data show that mammalian target of rapamycin (mTOR) inhibitors such as rapamycin, temsirolimus and everolimus, may be potential target therapies for MM patients, especially if associated with other drugs [3-5]. mTOR is a serine-threonine protein kinase that belongs to the phosphoinositide 3-kinase (PI3K)-related kinase family. It plays a central role in regulating critical cellular processes such as growth, proliferation, cytoskeletal organization, transcription, protein synthesis and ribosomal biogenesis [6, 7]. In mammals, there are two mTOR protein complexes: the regulatory associated protein of mTOR (Raptor)-G protein β-subunit-like protein (GβL)-mTOR complex (mTORC1) and the rapamycin-insensitive companion of mTOR (Rictor)-GβL-mTOR complex (mTORC2). mTORC1 directly phosphorylates the two key translation regulators p70 ribosomal S6 kinase (P70S6K) and eukaryotic initiation factor 4E binding protein 1 (4E-BP1). Phosphorylation of P70S6K stimulates ribosome biogenesis while multi-site phosphorylation of 4E-BP1 results in its dissociation from elF4E thereby allowing elF4E to enhanced cap-dependent translation of oncogenic cap-mRNAs such as MYC, HIF-1 and Cyclin D1 [8-10]. Previous studies demonstrates hyper activation of mTOR in myeloma downstream of PI3-K/AKT [11, 12]. Moreover the PI3K/AKT/mTOR pathway is one of the major pathways mediating cytokine-induced MM cell proliferation, survival and development of drug resistance [12, 13]. The Rictor-GβL-mTOR complex (mTORC2) is relatively rapamycin-insensitive and has a key role in cell survival and proliferation through the AKT phosphorylation occurring on serine 473 [14]. The AKT S473 phosphorylation is relatively specific for MM tumour cells, as adjacent non-malignant hematopoietic cells in patient marrows are usually negative for S473 staining. MM cell line studies suggest that an additional TORC2 target, SGK (glucocorticoid-inducible kinase), is also up-regulated, as its substrate, NDRG1 (N-myc down regulated gene 1), is hyper phosphorylated. Moreover TORC2 is currently known to directly regulate protein kinase C-α (PKC- α) [6, 15].
Consistent with its primary target being the translation machinery, mTOR is predominantly localized in the cytoplasm, associated with a variety of intracellular membrane structures [16, 17]. However, a nuclear localization of mTOR has been initially found in rhabdomyosarcomas (Rh30 and Rh41), human fibroblasts (IMR90) and HCT8 colon carcinoma cells [18]. Furthermore, mTOR becomes nuclear in HEK293 cells treated with leptomycin B, a specific inhibitor of nuclear export receptor Crm1, suggesting that mTOR is a cytoplasmic-nuclear shuttling protein [19]. Pomalidomide is a novel IMID® immunomodulatory drug approved for the treatment of MM by FDA in February 2013. Immunomodulatory drugs (thalidomide, lenalidomide and pomalidomide) possess several activities: immunomodulatory (by enhancing expansion of NKT cells and by reducing regulatory T cell–Tregs-); antiangiogenetic (by decreasing the expression of VEGF and bFGF, thereby inhibiting new blood vessel formation); anti-inflammatory (by inhibiting the production of TNFα); anti-proliferative effects (by inhibiting the activity of NF-kB, C/EBPβ and CDKs) [20]. Cereblon (CRBN) has been considered one of the immunomodulatory drugs target proteins: CRBN expression is required for lenalidomide activity and its deregulation confers resistance to drug treatment [21]. In vitro studies showed that pomalidomide is 10-fold more potent than lenalidomide in inhibiting TNFα; pomalidomide has distinct mechanisms of action compared with lenalidomide including direct anti-proliferative (by up-regulation the expression of p21 WAF1 tumor suppressor gene) and pro-apoptotic effects (by enhancing MM sensitivity to Fas-induced and TRAIL/Apo2L-induced apoptosis via a caspase-8-dependent mechanism) [22]. A recent phase 1 trial suggests the potential of lenalidomide-everolimus combination therapy in relapsed/refractory MM patients [23]. This combination is based on preclinical studies showing synergistic activity of mTOR inhibitors with lenalidomide and their ability to overcome the protective effects of growth factors in the myeloma tumour milieu [4]. The molecular mechanism by which these drugs interfere seems to include the mitogen-activated protein kinase (MAPK) and the PI3K/AKT kinase pathways but is not known completely.
The aim of this work is to study the activation of the AKT/mTOR/P70S6K/4E-BP1 pathway and its prognostic impact in MM patients. We also evaluate cellular localization of mTOR protein in MM cell lines and in primary tumour cells. Moreover the role of the pomalidomide in regulating the mTOR pathway is analysed.
RESULTS
Effect of pomalidomide on tumour cell proliferation and apoptosis
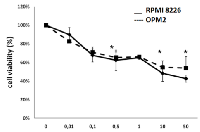
OPM2 and RPMI8226 cell lines were cultured at 24h and 48h and incubated with increasing doses of pomalidomide (ranging from 0.01 μM to 50 μM). MTT assay demonstrates that pomalidomide significantly reduced cell viability of RPMI8226 and OPM2 cells at 48h with IC50 values of 8 μM and 10 μM, respectively (FIG 1).
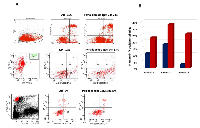
The apoptotic effect of pomalidomide was evaluated on MM cell lines and patients’ MM cells by flow cytometry. MM cell lines were incubated with Pomalidomide 0.01, 0.1, 1, 10 and 50 μM at 24h, 48h and 72h. Plasmacells were labelled with annexin V conjugated with fluorescein isothiocyanate and propidium iodide and annexin V+ /PI- cells were considered in early apoptosis phase. No significant apoptosis was detected in RPMI8226 and OPM2 cells (data not shown). Plasmacells from three MM patients were identified using anti-CD38 antibody and incubated with pomalidomide 1 μM for 24h: pomalidomide significantly induced apoptosis cell death (23%, 33% and 26% versus controls 11%,18%,3%, P<0.05) (FIG 2).
Localization of mTOR protein by confocal microscopy
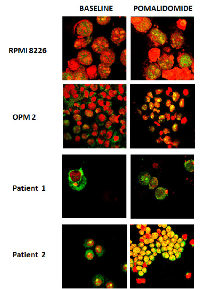
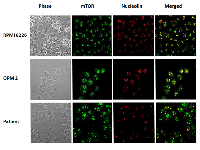
Immunofluorescence assays using antibodies against mTOR protein were performed on RPMI8226 and OPM2 cell lines and on CD138 positive cells from thirteen MM patients. We evidenced that in RPMI8226 and OPM2 cells, the mTOR protein is distributed throughout the cell cytoplasm and nucleus at baseline. After incubation with pomalidomide 10 μM for 48 h, MM cell lines demonstrated an increase of the nuclear mTOR protein (FIG 3). CD138+ cells from four multiple myeloma patients were analyzed at baseline and after pomalidomide treatment 1 μM for 24 h. Nuclear mTOR localization was detected in three out four cases at baseline. An increase of the nuclear mTOR protein after pomalidomide treatment was detected in three patients: two of them had a nuclear mTOR localization at baseline while the remaining patient acquired nuclear mTOR localization after pomalidomide treatment (FIG 3). We compared mTOR and nucleolin co-localization in RPMI8226 and OPM2 cells and in CD138 positive cells from nine MM patients. MM cells exhibit varying staining patterns with the mTOR antibody: the nuclear patterns included punctate bodies, small dot-like speckles and speckles. On the same cells, the nucleolin antibody stained nucleoli and some dot-like speckles. The co-localization of mTOR protein (green) and nucleolin (red) occurred frequently in nucleoli (which are phase dense in FIG 4) and in some nuclear speckles, and exhibited merged (yellow) regions.
Immunohistochemistry
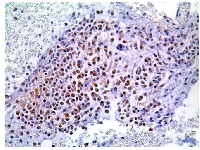
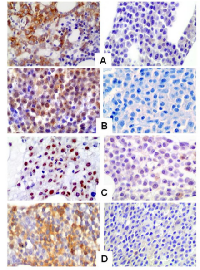
P-mTOR staining was analyzed on bone marrow sections from 101 MM patients. Globally, 57 out 101 (56.4%) cases demonstrated p-mTOR positivity with a predominant cytoplasmic staining pattern. A nuclear p-mTOR staining was also demonstrated in 14 out 101 cases analyzed (13.9%) (FIG 5). All but one patient showed both nuclear and cytoplasmic p-mTOR staining. Immunohistochemistry for p-AKT, p-P706SK and p-4E-BP1 were also performed on 70 MM cases on the basis of the sample availability. The median cut-off value was chosen to identify those patients who stained positive for each antibody. Specimens with an HSCORE of ≥30 and ≥45 were classified as p-mTOR (range 0-285) and p-AKT (range 0-285) positive while for p-P70S6K and p-4E-BP1 specimens were considered positive when the HSCORE was ≥60 (range 0-285) and ≥40 (0-270), respectively. Globally, 44 out 70 (62.8 %) MM patients stained positive for p-mTOR and 37 (52.8%), 42 (60%) and 38 (54.3%) cases stained positive for p–AKT, p-P706SK and p-4E-BP1, respectively.
P-mTOR and p-AKT are expressed in MM patients with a predominance of cytoplasmic staining pattern; p-P70S6K and p-4E-BP1 are also detected with nuclear staining pattern (FIG 6).
Overall, p-mTOR expression significantly correlates with p-AKT (concordance r=0.29, P=0.05), p-P70S6K (r=0.35, P=0.001), and p-4E-BP1 (r=0.41, P=0.0001) positive staining consistent with the hypothesis that the mTOR pathway is activated in a subset of MM patients.
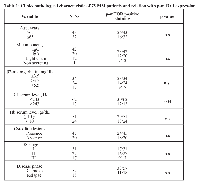
Clinical data were available on 75 MM patients (Table 1) (57 newly diagnosed and 18 relapsed disease) as follow: median age 66 years (range 38-87); monoclonal component IgG 42 cases (56%), IgA 20 cases (26.7%), 12 light chain myelomas (16%) and 1 non-secreting myeloma (1.3%); LDH serum levels higher than normal, 13 patients (17.3%); serum levels of β2-microglobulin higher than 3.5 mg/dL and 5.5 mg/dL 24 (32%) and 17 (22.6%) cases, respectively; Hb <10 gr/dL 24 patients (32%); osteolytic lesions 45 cases (60%); ISS stage I, II, III in 31 (41.4%), 27 (36%) e 17 (22.6%) patients, respectively.
Statistical correlation between p-mTOR expression and clinical variables clinical data
Clinical data were available on 75 MM patients (57 newly diagnosed and 18 relapsed disease) as follow: median age 66 years (range 38-87); monoclonal component IgG 42 cases (56%), IgA 20 cases (26.7%), 12 light chain myelomas (16%) and 1 non-secreting myeloma (1.3%); LDH serum levels higher than normal, 13 patients (17.3%); serum levels of β2-microglobulin higher than 3.5 mg/dL and 5.5 mg/dL 24 (32%) and 17 (22.6%) cases, respectively; Hb <10 gr/dL 24 patients (32%); osteolytic lesions 45 cases (60%); ISS stage I, II, III in 31 (41.4%), 27 (36%) e 17 (22.6%) patients, respectively.Cytoplasmic p-mTOR positive staining (HSCORE ≥30) correlates with high LDH serum levels (P=0.004, chi-square 8.4). These data suggest that the expression of m-TOR protein in MM patients may have clinical and prognostic implications.
Western blotting
Cytoplasmic and nuclear distribution of mTOR and p-mTOR was investigated by western blot in OPM2 and RPMI8226 cells. Cytoplasmic and nuclear fractions were obtained following specific protocol described below. Western blot analysis revealed that mTOR and p-mTOR can be detected in the cytoplasm and in the nucleus at baseline in both myeloma cell lines. As expected, the mTOR and p-mTOR protein levels were significantly higher in the cytoplasm when compared to the nucleus. Treatment with pomalidomide 10 μM for 48h, increased nuclear mTOR and p-mTOR protein levels in the nucleus with a concomitant reduction of the cytoplasmic mTOR fraction in RPMI8226 and OPM2 cells. The p-mTOR cytoplasmic fraction was reduced in RPMI8226 but was variable in OPM2 cells (Figure 7 A,B). Pomalidomide 10 μM did not affect AKT and p-AKT S473 protein levels while perifosine (20 μM), as expected, reduced p-AKT S473 levels in OPM-2 cells (Figure 8). RPMI 8226 did not demonstrate constitutive activation of p-AKT (data not shown).
DISCUSSION
In this study, we found cytoplasmic p-mTOR expression in almost half of the multiple myeloma samples investigated by immunohistochemistry. Moreover, p-mTOR expression was significantly correlated with the expression of p-AKT, p-P70S6K, and p-4E-BP1, suggesting an activation of the mTOR pathway in a subset of MM patients.
Early studies identified activation of the P13K/AKT pathway in MM cell lines and in primary MM cells [24-26]. Shi et al [11, 12] demonstrated that IL-6 or IGF-1 exposure up-regulated phosphorylation of p70S6K and 4EBP-1 and that the mTOR inhibitors may prevent activation of the mTOR pathway by inhibiting cytokine-dependent myeloma cell growth. In addition to the stimulating effects of IL-6 and IGF-1, loss of function mutations of the tumour suppression gene PTEN in several MM cell lines, results in phosphorylation of the mTOR substrates [12]. More recent data suggest that in MM, RAS mutations could activate TORC1 and TORC2 complexes and this events was correlated with an aggressive phenotype [15, 27]. Our study suggests that in MM specimens mTOR activation correlates with AKT activation, and thus usually occurs downstream of PI3-K/AKT.Activation of the mTOR signalling pathway has recently been found to be strongly implicated in several human cancers and in age-related disease [28]. In early stage, triple negative (estrogen and progesteron receptor, Her2 amplification) breast carcinomas, phosphorylated mTOR expression significantly correlated with worse overall survival and recurrence-free survival [29]. In oesophageal squamous cell carcinoma, high levels of phosphorylated mTOR were significantly associated with shortened disease specific survival and mTOR expression remained an independent adverse prognostic factor in multivariate analysis [30]. Similar data were obtained in early stage Non Small Cell Lung Cancer where angioinvasion and mTOR expression were significant predictors of poor survival at both univariate and multivariate analysis [31]. The relevance of mTOR signalling in Renal Cell Carcinoma (RCC) is highlighted by the success in using mTOR inhibitors (temsirolimus and everolimus) to treat patients with advanced disease [32]. A recent work suggests that in clear RCC a cumulative number of altered biomarkers in mTOR pathway (p-AKT, p-P706SK, p-mTOR, HIF-1alfa, Raptor, PTEN, P13K, p-4EBP-1) correlates with aggressive tumour biology (tumour stage and grade) and inferior disease outcome [33]. Our study also demonstrated that phosphorylated mTOR expression, as detected by immunohistochemistry, was significantly correlated with high LDH serum levels. Recent data highlighted the importance of this variable in high risk myeloma. A new definition of this latter category is ongoing and it includes, in addition to unfavourable cytogenetic profile [t(4;14) and/or del(17p)], International Staging System 3 (β2microglobulin >5.5 gr/dL) and high LDH serum levels [34].
Activation of the PI3K/AKT/mTOR pathway is frequently implicated in resistance to anticancer therapies, including tyrosine kinase inhibitors, radiation, and cytotoxic drugs. Moreover preclinical evidence shows that inhibitors of PI3K or mTOR can restore sensitivity in breast cancer, non-small-cell lung cancer and glioblastoma cells resistant to biologic and cytotoxic drugs [35]. The possibility that p-mTOR expression may confer adverse prognosis in MM patients by activating its own pathway or by conferring resistance to drugs should be investigated.
mTOR protein localization is mainly cytoplasmic. However nuclear localization has been found in several tumors [36-38]. Here we found that in multiple myeloma cell lines and in primary myeloma cells mTOR is distributed throughout the cell cytoplasm and also nucleus at baseline. By immunofluorescence and confocal microscopy, cytoplasmic and nuclear distribution of mTOR was detected in RPMI8226 and OPM2 cell lines and in plasma cells from 12 out 13 MM patients, as also confirmed by co-localization experiments with the nuclear protein nucleolin. A nuclear p-mTOR staining was also demonstrated in 14 out 101 cases (13.9%) by immunohistochemistry. Results from western blot analysis on RPMI8226 and OPM2 cells revealed that mTOR and p-mTOR can be detected in both the cytoplasm and the nucleus. This is the first report of a mTOR nuclear localization in multiple myeloma. The nuclear distribution of mTOR is surprising and intriguing, as this pathway regulates cytoplasmic targets. In Saccharomyces cerevisiae the mTOR kinase localizes to tRNA and 5S rRNA genes and, by interacting with the transcriptional factor TFIIIc, recognizes the promoter of this genes. Moreover mTOR can phosphorylates Maf1, a repressor that binds and inhibits RNA pol III, therefore enhancing its inhibitor function [39]. In mammals, a previous work suggests that in HEK 293 cells mTOR binds to the promoters of RNA polymerase I- and III-transcribed genes and that this association is regulated by growth signals and is sensitive to rapamycin [40]. In our work, in MM cells, mTOR was mainly localized in the nucleolus. As its primary function is to transcribe and modify rRNA and to assembly ribosomes, this localization may suggest a role in regulating such functions.
Treatment with pomalidomide performed by western blot, also increased nuclear mTOR protein levels in RPMI8226 and OPM2 cells with a concomitant reduction of the cytoplasmic fraction. We also observed a cytoplasmic-nuclear shuttling of the p-mTOR protein with a cytoplasmic fraction reduction in RPMI8226 cells and a variable amount in OPM2 cells. This work adds more information about pomalidomide mechanisms of action in multiple myeloma. A previous study analysed cytoplasmic-nuclear shuttling of mTOR protein in both human embryonic kidney (HEK) 293 cells and monkey kidney epithelial CV-1 cells [19]. By using leptomicycin B, a specific inhibitor of nuclear export receptor Crm1, authors demonstrated that mTOR is a cytoplasmic-nuclear shuttling protein. Moreover, inhibition of mTOR nuclear export by leptomicin and the addition of exogenous nuclear import signals to mTOR, coincides with diminished p70S6K activation and 4E-BP1 phosphorylation. It should be possible that nuclear shuttling of the mTOR protein in MM cells, as induced by pomalidomide, may regulate the mTOR pathway both by diminished the cytoplasmic p-mTOR fraction and by direct regulation of the protein synthesis machinery via extracellular signals.
Previous studies on the anti-proliferative effects of these compounds suggested that they may inhibit the activity of PI3K/AKT pathway. Lenalidomide is able to inhibit the phosphorylation of AKT in response to growth factors in endothelial cells that correlates with anti-migratory and anti-angiogenic effect [41]. Furthermore lenalidomide significantly attenuates VEGF-induced AKT phosphorylation in endothelial cells thus interfering with endothelial cell survival, migration and vessel formation [42]. In our work pomalidomide does not affect directly AKT S473 phosphorylation status in OPM 2 cells suggesting that the drug is not active on mTORC 2. Recent data highlight that phosphorylation of AKT at S473 by mTORC2 is necessary for full AKT activation [43] so we can suppose that pomalidomide activity on mTOR and p-mTOR proteins may occur independently from AKT.
More studies are needed to evaluate the significance of nuclear mTOR localization, transport and function in MM. Our work suggests a way to identify a subgroup of MM patients with mTOR pathway activation in order to select patients that may benefit from a target therapy. Moreover, a better knowledge about pomalidomide mechanisms of action may explain its efficacy in the treatment of MM patient when used either alone or in combination with other drugs.
Pomalidomide activity on the mTOR activation status may also suggest pomalidomide-mTOR inhibitor combinations in order to enhance drugs activity and efficacy in MM and solid tumors too.
MATERIALS AND METHODS
Cell lines
RPMI8226 were kindly provided by Dr Roberto Piva ( Department of Pathology and CeRMS, University of Turin). OPM2 was purchased from DSMZ (Braunschweig, Germany). All MM cell lines were cultured in IMDM (Sigma-Aldrich Corp. St. Luis, MO, USA) containing 10% fetal bovine serum, 2mM L-glutamine (Gibco-Invitrogen, Grand Island, NY), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco).
Drugs
Pomalidomide was obtained from Celgene. The drugs were dissolved in dimethylsulfoxide (DMSO; Sigma) at a concentration of 200 mM and were stored at –20°C until use. Pomalidomide was diluted in culture medium (0,01-50 μM) with less 0,1% DMSO immediately before use. Perifosine was supplied by Selleckchem, dissolved in DMSO <1mg/ml and stored at -80°C until use.
MTT colorimetric survival assay
OPM2 and RPMI8226 cell lines were plated in 96-wells plates for 24h and then treated with increasing concentrations of pomalidomide (ranging from 0.01 μM to 50 μM) for 24 h and 48 h, respectively. A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma-Aldrich Corp. St. Luis, MO, USA) assay was performed according to the manufacturer’s instructions. Assorbance was measured at 570 nm (with correction using readings at 690 nm) using a microplate reader (Asys UVM 340). The cytotoxicity was expressed as the percentage of cells surviving relative to untreated cultures. The half maximal inhibitory concentration (IC50 values) was calculated using the ED50 Plus v1.0 online software. All experiments were repeated at last 3 times, and each experimental condition was performed in triplicate. Data are presented as mean ±SD.
Apoptosis assay
Apoptosis was evaluated by flow cytometry for the detection of annexin V-positive cells. MM cell lines were incubated for 24 h, 48 h and 72 h with media alone or with varying concentration of pomalidomide ( 0.01, 0.1, 1, 10 and 50 μM).
Bone marrow aspirates were subjected to Ficoll Hypaque gradient centrifugation and mononuclear cells were suspended in IMDM media containing 20% fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were placed at in a plate at a concentration of 2 X 106 cells/mL; pomalidomide was added to the medium at the concentration 1 µM. Patient plasma cells were cultured for 24 h and then harvested. Plasma cells were identified by using anti-CD38 antibody (Becton Dickinson, San Jose Ca). Apoptosis was quantified using Annexin V-FITC/Propidium iodide apoptosis detection kit, as per manufacturer’s instructions (Immunostep, Salamanca, Spain), followed by an analysis on FACS Canto II, (Becton Dickinson, San Jose Ca).
Confocal Microscopy
RPMI8226 and OPM2 cells were incubated with 10 µM of pomalidomide for 48 h. Bone marrow samples of MM patients were collected during standard procedure after informed consent and subjected to Ficoll-Hypaque gradient centrifugation (Biochrom, Berlin, Germany). The plasma cell populations were positively selected by the immunomagnetic method using CD138 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, 10 μL of CD138 microbeads and 90 μL of buffer M (PBS, pH 7.2, bovine serum albumin 0.5%, ethylenediaminetetraacedic acid 2 mmol/L) for 5×106 mononuclear cells were added and incubated for 15 minutes at 4°C. After washing, the cells were layered on a VarioMACS separation column (Miltenyi Biotec) following the manufacturer’s instructions. CD138 positive cells were removed from the column, and their purity (90%) was assessed by cell morphology. Plasma cells were incubated with 1 µM of pomalidomide for 24 h.
Slides from MM cell lines and primary MM cells were subjected to immunofluorescence with mTOR antibodies (Abcam, Cambridge, UK) at 1:100 dilution for 2 h at room temperature and washed extensively in PBS. Antibody–antigen complexes were detected by incubation for 60 min with a goat anti-rabbit Alexa Fluor 488-conjugated secondary antibody (Jackson Immuno Research Laboratories, Baltimora Pike West Grove PA). Slides were washed in PBS and treated with propidium iodide (Sigma-Aldrich Corp. St. Luis, MO, USA) for 5 min to stain the nucleus. Cells were analyzed with a Confocal and Fluorescence Microscopy Lab (Zeiss LSM 510; Axiovert 200 with cd camera and computer-driven image acquisition; semi-automated Eppendorf micro-manipulator) equipped with a 40X Plan Neofluar objective. To confirm the nuclear localization of mTor we perform a Z stack of 9-20 slice with an interval of 0.5 μm. For nucleolin staining, C23 (H-6), a mouse monoclonal antibody was used at 1:100 dilution (Santa Cruz Biotechnology, California) and the antibody-antigene complexes were detected by incubation for 60 min with a goat anti-mouse Alexa Flour 568-conjuated secondary antibody.
Immunohistochemistry and patients
Immunohistochemical studies was conducted on bone marrow biopsies of 101 symptomatic MM patients fulfilling the International Myeloma Working Group Diagnostic Criteria [44]. The selection was based on the availability of bone marrow biopsies plus clinical data. Bone marrow aspirates and biopsies were taken from each patient at the same time during standard diagnostic procedure after informed consent. Clinical data of MM patients are summarized in Table 1. Serial section to that stained with hematoxylin and eosin were collected onto charged slides for immunohistochemistry. An automated immunoperoxidase procedure was employed (Autostainer, DakoCytomation, Glostrup, Denmark) using standard detection methods based on biotin-free reagents (peroxidase conjugated dextran polymers; EnVisionTM + System/HRP,Rabbit/Mouse, DakoCytomation). Before starting the immune reaction, endogenous peroxidase activity was blocked with hydrogen peroxide, and antigen retrieval was obtained by microwaving (0.1 M citrate buffer, pH 6.0) for all antibodies tested. Diaminobenzidine was used as chromogen to reveal immune reaction.
Neoplastic plasma cells were counted on slides immunostained with CD138 antibody (clone MI 15, diluition 1:150; Dako Cytomation), rabbit polyclonal Kappa (diluition 1:50000, Ylem, Rome, Italy) and rabbit polyclonal Lambda (diluition1: 50000, Ylem).
The primary antibodies tested were rabbit monoclonal anti p-mTOR (Ser2448) (clone 49F9, diluition 1:50; Cell Signaling Technology, Beverly, MA), rabbit monoclonal anti p-AKT (Ser473) (clone 736E11, diluition 1:40; Cell Signaling), rabbit monoclonal anti p-4E-BP1(Thr37/46) (clone 236B4, diluition1:350; Cell Signaling), mouse monoclonal anti p-P70S6-Kinase (Thr389) (clone 1A5, diluition 1:300; Cell Signaling).
The slides were counterstained with Mayer hematoxylin and evaluated with a Leica DMD 108 Digital Microimaging Device (Leica Microsystems, Milan, Italy).
Slides were scored by two independent observers and all cases were analyzed using a semi-quantitative histologic score method, as described in the literature [45]. Briefly, immunostaining intensity of each case was semi-quantitatively scored as follows: 0, no staining ; 1,weak staining; 2, moderate staining; and 3, strong staining. In addition, the percentage of neoplastic cells was evaluated. For each case, a value designated HSCORE was obtained multiplying each intensity by the corresponding percentage of positive cells [HSCORE =Σ(IXPC), where I and PC represent intensity and percentage of cells, respectively].
The expression level of each marker was determined by counting all plasma cells in each bone marrow section. Both the nucleus and the cytoplasm were evaluated for each antibody.
The immunostaining for mTOR pathway molecules were performed using antibodies validated in a previous study on colon cancer [46]. and their specificity was further tested by means of western blot analysis. Negative controls were obtained by omitting the primary antibody on a parallel section of each immunohistochemical run.
Western Blotting
RPMI8226 and OPM2 cells were incubated with 10 µM of pomalidomide for 48 h and western Blot was performed on nuclear and cytoplasmatic fractions.Cells were washed twice with ice-cold PBS and pellet was lysed in cytoplasmic extraction buffer containing 10 mM HEPES, pH 7.9, 10 mM KCl, EDTA 0.1 mM, supplemented with protease inhibitors (1 µ g/ml aprotinin, 1 µ g/ml pepstatin, 1 µ g/ml leupeptin) and 1 m M sodium orthovanadate and incubated for 2 min at room temperature and another 10 min on ice.
Disruption of the cell membrane was achieved by the addition of CHAPS (3-[(3-cholamidopropyl)dimetthylammonio]-1-propanesulfonate) stock solution (10x), at final concentration of 0.6% [47].
Gently mix and broken cells are then homogenized by passing them through a 19-g needle for three times. Cytoplasmic extract was separated by centrifugation at 4°C and 14000 RPMI for 5 min and was stored at -80°C. The remaining pellet contains nuclei was washed three times in extraction buffer supplemented with 0.6% CHAPS and then centrifugated. The nucleic pellet was lysed in nuclear extraction buffer ( 20 mM HEPES, pH 7.9, 400 mM KCl, 1 mM EDTA, 1mM EGTA, 10% glycerol, DTT 1 mM ,1 m M sodium orthovanadate), supplemented with protease inhibitors and incubated for 20 min on ice. Supernatant containing soluble nucleic proteins was collected by centrifugation at 4°C and 14000 RPMI for 15 min and stored at –80°C.
Nuclear and cytoplasmic fractions were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred to nitrocellulose by elettrophoresis for 1-2 h. Non-specific binding was blocked by incubation with BSA 5% at room temperature for 1 h and membranes were incubated overnight with primary antibody against mTOR and p-mTOR (1:500, Abcam, Cambridge, UK) at 4°C. The membranes were washed four times with TBS-Tween 0,3%, incubated with secondary antibody (1:1000) at room temperature for 1 h, and again washed four times with TBS-Tween 0,3%. The membrane was stripped and reprobed with anti-vinculin, anti-laminin and anti-tubulin antibodies (1:1000)(Santa Cruz Biotechnology, California) to ensure equivalent protein loading. Immunoreactive bands were visualized using Chemidoc TM XRS + with Image Lab TM software molecular imager (Biorad).
Cell lines were treated with Perifosine 20µM for 6 h, 12 h, 24 h and 48 h. Total cell extract was obtained by incubating of cells for 30 min on ice with Ripa Buffer (50 mMTris HCl, 150 mM NaCl, 1% Triton X-100, 10% SDS, 1% Deoxycholic acid, 1 µ g/ml aprotinin, 1 µ g/ml pepstatin, 1 µ g/ml leupeptin and 1 m M sodium orthovanadate). Total extract was separated by centrifugation at 4°C, 13000 RPMI for 10 min andstored at -80°C. Cell lysates were subjected to SDS-page, transferred to nitrocellulose membrane, and immunoblotted with antibodies against AKT and p-AKT (Ser473) (1:1000) and Tubulin (Santa Cruz Biotechnology, California) [48, 49].
Statistical analysis
Statistical significance of differences observed in drug-treated versus control cultures was determined by using Student t test. The minimal level of significance was considered P <.05. Data was analyzed using GraphPAD Prism software (version 5) and ED50 Plus v1.0 online software.
Fisher exact or chi-square tests were used to test for differences among levels of categoric variables between patients with and without p-mTOR. Spearrman’s Rank correlation coefficient was used to evaluate the correlation between p-mTOR, p-AKT, p-P706SK and p-4E-BP1expression. Analysis were performed using the SPSS software package (SPSS, Chicago, IL).
ACKOWLEDGEMENTS
The authors would like to thank doctor Gianfranco Fonte for the statistical analysis and doctor Andrea Grua for the image processing.
CONFLICT OF INTEREST
The authors have not conflicts of interest to disclose.
GRANT SUPPORT
This work was supported by Celgene Corporation.
- 1. European perspective on multiple myeloma strategies: update following recent congress. Oncologist. 2012; 17(5):592-606. https://doi.org/10.1634/theoncologist.2011-0391. [PubMed].
- 2. San Miguel J, Weisel K, Moreau P, Lacy M, Song K, Delforge M, Karlin L, Goldschmidt H, Banos A, Oriol A, Alegre A, Chen C, Cavo M, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013; 14(11):1055-1066. https://doi.org/10.1016/S1470-2045(13)70380-2. [PubMed].
- 3. Mechanism by which mammalian target of rapamycin inhibitors sensitize multiple myeloma cells to dexamethasone-induced apoptosis. Cancer Res. 2006; 66(4):2305-2313. https://doi.org/10.1158/0008-5472.CAN-05-2447. [PubMed].
- 4. Raje N, Kumar S, Hideshima T, Ishitsuka K, Chauhan D, Mitsiades C, Podar K, Le Gouill S, Richardson P, Munshi NC, Stirling DI, Antin JH, Anderson KC. Combination of the mTOR inhibitor rapamycin and CC-5013 has synergistic activity in multiple myeloma. Blood. 2004; 104(13):4188-4193. https://doi.org/10.1182/blood-2004-06-2281. [PubMed].
- 5. Combination of mTOR inhibitor rapamycin and HSP90 inhibitor 17 allylamino 17-demetoxiygeldanamycin has synergistic activity in multiple myeloma. Clin Cancer Res. 2006; 12:6826-6835. https://doi.org/10.1158/1078-0432.CCR-06-1331. [PubMed].
- 6. The mammalian target of rapamycin pathway as a therapeutic target in multiple myeloma. Leukemia and Lymphoma. 2011; 52(10):1857-1866. https://doi.org/10.3109/10428194.2011.580478. [PubMed].
- 7. Dissecting the role of mTOR: lessons from mTOR inhibitor. Biochimica et Biophisica Acta. 2010; 1804: 433-439. https://doi.org/10.1016/j.bbapap.2009.12.001. [PubMed].
- 8. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trend Biochem Sci. 2006; 31:342-348. https://doi.org/10.1016/j.tibs.2006.04.003. [PubMed].
- 9. Upstream and downstream of mTOR. Genes Dev. 2004; 18:1926-1945. https://doi.org/10.1101/gad.1212704. [PubMed].
- 10. mTor, translation, initiation and cancer. Oncogene. 2006; 25:6416-6422. https://doi.org/10.1038/sj.onc.1209888. [PubMed].
- 11. Signal pathways involved in activation of p70S6K and phosphorylation of 4E-BP1 following exposure of multiple myeloma cells to IL-6. J Biol Chem. 2002; 277:15712-15720. https://doi.org/10.1074/jbc.M200043200. [PubMed].
- 12. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 2002; 62(17):5027-5034. [PubMed].
- 13. Identification and validation of novel therapeutic targets for multiple myeloma. J Clin Oncol. 2005; 23(26):6345-6350. https://doi.org/10.1200/JCO.2005.05.024. [PubMed].
- 14. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of AKT/PKB by the rictor-mTOR complex. Science. 2005; 307(8):1098-1101. https://doi.org/10.1126/science.1106148. [PubMed].
- 15. Targeting TORC2 in multiple myeloma with a new mTOR kinase inhibitor. Blood. 2010; 116:4560-4568. https://doi.org/10.1182/blood-2010-05-285726. [PubMed].
- 16. FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc Natl Acad Sci U S A. 2002; 99(7):4319-4324. https://doi.org/10.1073/pnas.261702698. [PubMed].
- 17. FKBP12-Rapamycin-associated protein or mammalian target of rapamycin (FRAP/mTOR) localization in the endoplasmic reticulum and the Golgi apparatus. J Biol Chem. 2004; 279(1):772-778. https://doi.org/10.1074/jbc.M305912200. [PubMed].
- 18. Predominant nuclear localization of mammalian target of rapamycin in normal and malignant cells in culture. J Biol Chem. 2002; 277(31):28127-28134. https://doi.org/10.1074/jbc.M202625200. [PubMed].
- 19. Kim JE, Chen J. Cytoplasmic-nuclear shuttling of FKBP12-rapamycin associated protein is involved in rapamycin-sensitive signaling and translation initiation. PNAS. 2000; 97(26):14340-14345. https://doi.org/10.1073/pnas.011511898. [PubMed].
- 20. Mechanism of immunomodulatory drugs’ action in the treatment of multiple myeloma. Acta Biochim Biophys Sin. 2014; 46(3):240-253. https://doi.org/10.1093/abbs/gmt142. [PubMed].
- 21. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011; 118(18):4771-4779. https://doi.org/10.1182/blood-2011-05-356063. [PubMed].
- 22. Pomalidomide: new immunomodulatory agent with potent antiproliferative effects. Crit Rev Oncol Hematol. 2013; 88 Suppl1:S36-44. https://doi.org/10.1016/j.critrevonc.2013.02.001. [PubMed].
- 23. Outcomes in patients with relapsed or refractory multiple myeloma in a phase I study of everolimus in combination with lenalidomide. Br J Haematol. 2014; 166(3):401-409. https://doi.org/10.1111/bjh.12909. [PubMed].
- 24. The phosphatidylinositol 3-kinase/AKT kinase pathway in multiple myeloma plasma cells: roles in cytokine-dependent survival and proliferative responses. Cancer Res. 2000; 60:6763-6770. [PubMed].
- 25. The AKT kinase is activated in multiple myeloma tumor cells. Blood. 2001; 98:2853-2855. https://doi.org/10.1182/blood.v98.9.2853. [PubMed].
- 26. Loss of PTEN expression leading to high activation in human multiple myelomas. Blood. 2000; 96:3560-3658. [PubMed].
- 27. Downstream effectors of oncogenic RAS in multiple myeloma cells. Blood. 2003; 101:3126-3135. https://doi.org/10.1182/blood-2002-08-2640. [PubMed].
- 28. mTOR is a key modulator of ageing and age-related disease. Nature. 2013; 493:338-345. https://doi.org/10.1038/nature11861. [PubMed].
- 29. Phosphorylated mTOR expression correlates with poor outcome in early-stage triple negative breast carcinomas. Int J Clin Exp Pathol. 2012; 5(8):806-813. [PubMed].
- 30. Clinicopathologic significance and function of mammalian target of rapamycin activation in oesophageal squamous cell carcinoma. Hum Pathol. 2013; 44(2):226-236. https://doi.org/10.1016/j.humpath.2012.05.011. [PubMed].
- 31. Overexpression of the mammalian target of rapamycin (mTOR) and angioinvasion are poor prognostic factors in early stage NSCLC: a verification study. Lung Cancer. 2012; 75(2):217-222. https://doi.org/10.1016/j.lungcan.2011.06.012. [PubMed].
- 32. Phase II trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J Clin Oncol. 2010; 28(13):2131-2136. https://doi.org/10.1200/JCO.2009.26.3152. [PubMed].
- 33. Cumulative number of altered biomarkers in mammalian target of rapamycin pathway is an independent predictor of outcome in patients with clear renal cell carcinoma. Urology. 2013; 81(3):581-586. https://doi.org/10.1016/j.urology.2012.11.030. [PubMed].
- 34. Moreau P, Cavo M, Sonneveld P, Rosinol L, Attal M, Pezzi A, Goldschmidt H, Lahuerta JJ, Marit G, Palumbo A, van der Holt B, Bladé J, Petrucci MT, et al. Combination of International Scoring System 3, High Lactate Dehydrogenase, and t(4;14) and/or del(17p) Identifies Patients With Multiple Myeloma (MM) Treated With Front-Line Autologous Stem-Cell Transplantation at High Risk of Early MM Progression-Related Death. J Clin Oncol. 2014; 32(20):2173-2180. https://doi.org/10.1200/JCO.2013.53.0329. [PubMed].
- 35. Burris HA 3rd. Overcoming acquired resistance to anticancer therapy: focus on the PI3K/AKT/mTOR pathway. Cancer Chemother Pharmacol. 2013; 71 (4):829-842. https://doi.org/10.1007/s00280-012-2043-3. [PubMed].
- 36. Relation between outcome and localization of p-mTOR expression in gastric cancer. British J Cancer. 2009; 100:782-788. https://doi.org/10.1038/sj.bjc.6604915. [PubMed].
- 37. Localisation of phosphorylated mTOR expression is critical to tumour progression and outcomes in patients with endometrial cancer. European J Cancer. 2010; 46:3445-3451. https://doi.org/10.1016/j.ejca.2010.09.004. [PubMed].
- 38. Morphoproteomics demonstrates activation of mammalian target of rapamycin pathway in papillary thyroid carcinomas with nuclear translocation of MTOR in aggressive histological variants. Modern Path. 2011; 24:1553-1559. https://doi.org/10.1038/modpathol.2011.121. [PubMed].
- 39. mTOR associates with TFIIIC, is found at tRNA and 5S rRNA genes, and targets their repressor Maf1. Proc Natl Acad Sci U S A. 2010; 107(26):11823-11828. https://doi.org/10.1073/pnas.1005188107. [PubMed].
- 40. mTOR binds to the promoters of RNA polymerase I-and III-transcribed genes. Cell Cycle. 2010; 9(5):953-957. https://doi.org/10.4161/cc.9.5.10876. [PubMed].
- 41. Dredge K, Horsfall R, Robinson SP, Zhang LH, Lu L, Tang Y, Shirley MA, Muller G, Schafer P, Stirling D, Dalgleish AG, Bartlett JB. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005; 69(1-2):56-63. https://doi.org/10.1016/j.mvr.2005.01.002. [PubMed].
- 42. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc Res. 2009; 77(2):78-86. https://doi.org/10.1016/j.mvr.2008.08.003. [PubMed].
- 43. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature. 2014; 508(7497):541-545. https://doi.org/10.1038/nature13079. [PubMed].
- 44. International Myeloma Working Group: Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003; 121:749-757.
- 45. Correlation of reduction in MPR-1/CD9 and KA11/CD82 expression with recurrences in breast cancer patients. Am J Pathol. 1998; 153:973-983. https://doi.org/10.1016/s0002-9440(10)65639-8. [PubMed].
- 46. Co-expression of EGF receptor, TGFα and S6 kinase is significantly associated with colorectal carcinomas with distant metastases at diagnosis. Virchows Arch. 2007; 450:321–328. https://doi.org/10.1007/s00428-007-0370-2. [PubMed].
- 47. Cytoplasmic and nuclear distribution of the protein complexes mTORC1 and mTORC2: rapamycin triggers dephosphorylation and delocalization of the mTORC2 components rictor and sin1. Hum Mol Genet. 2008; 17(19):2934-2948. https://doi.org/10.1093/hmg/ddn192. [PubMed].
- 48. SNS-032 inhibits mTORC1/mTORC2 activity in acute myeloid leukemia cells and has synergistic activity with perifosine against Akt. J Hematol Oncol. 2013; 18:6-18. https://doi.org/10.1186/1756-8722-6-18. [PubMed].
- 49. Perifosine inhibits mammalian target of rapamycin signaling through facilitating degradation of major components in the mTOR axis and induces autophagy. Cancer Res. 2009; 69(23):8967-8976. https://doi.org/10.1158/0008-5472.CAN-09-2190. [PubMed].
Last Modified: 2016-06-04 18:45:03 EDT
