Oncoscience
Abstract | PDF | Full Text | Supplementary Materials | How to Cite
https://doi.org/10.18632/oncoscience.140
Epigenetic silencing of S100A2 in bladder and head and neck cancers
Juna Lee1, Piotr T. Wysocki2, Ozlem Topaloglu2, Leonel Maldonado2, Mariana Brait2, Shahnaz Begum5, David Moon2, Myoung Sook Kim2, Joseph A. Califano2,6, David Sidransky2,3, Mohammad O. Hoque2,3,4 and Chulso Moon1,2,3
1 Graduate Program in Human Genetics and Molecular Biology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
2 Department of Otolaryngology – Head and Neck Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
3 Department of Oncology and the Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
4 Department of Urology, Johns Hopkins University, Baltimore, Maryland, USA
5 Department of Pathology, Johns Hopkins University, Baltimore, Maryland, USA
6 Milton J. Dance Head and Neck Center. Greater Baltimore Medical Center, Baltimore, Maryland, USA
Correspondence to: Chulso Moon, email: [email protected]
Correspondence to: Mohammad Obaidul Hoque, email: [email protected]
Keywords: S100A2, methylation, head and neck cancer, bladder cancer, epigenetics
Received: January 29, 2015
Accepted: March 04, 2015
Published: March 16, 2015
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
S100A2, a member of the S100 protein family, is known to be downregulated in a number of human cancers, leading to its designation as a potential tumor suppressor gene. Here, we investigated the expression and methylation status of S100A2 in head&neck and bladder cancer. Reduced mRNA and protein expression was observed in 8 head&neck and bladder cancer cell lines. To explore the mechanism responsible for the downregulation of S100A2, we treated six cell lines with 5-aza-2’-deoxycytidine. We found S100A2 is silenced in association with aberrant promoter-region methylation and its expression is restored with 5-aza-2’-deoxycytidine treatment. Of 31 primary head&neck cancer cases and 31 bladder cancer cases, promoter methylation was detected in 90% and 80% of cases, respectively. Interestingly, only 1/9 of normal head&neck tissues and 2/6 of normal bladder tissues showed promoter methylation. S100A2 promoter methylation can be detected in urine and is more frequent in bladder cancer patients than in healthy subjects (96% vs 48% respectively). Moreover, increased methylation of S100A2 is linked to the progression of the tumor in bladder cancer (p<0.01). Together, this data shows that methylation-associated inactivation of S100A2 is frequent and may be an important event in the tumorigenesis of head&neck and bladder cancer.
INTRODUCTION
The S100 family is a large subgroup of Ca2+-binding proteins. There are at least 25 different members, displaying from 16% to 98% homology in sequence identity [1]. S100 proteins have been implicated in pleiotropic cellular events, with specific functions for each of the family members such as cell cycle regulation, cell growth, cell differentiation, and motility [1]. In addition, a range of different human diseases has been associated with changes in the expression of S100 genes, including cancer [2]. For example, S100B is known to be upregulated in melanomas and is used as a tumor marker [3]. Similarly, S100A4 is reported to be overexpressed in metastatic breast cancer cell lines and human tumor tissues [2]. Although many S100 genes are located in a cluster in chromosome 1q21 there is no evidence that their expression is synchronized by any manner. On the contrary, in a given cell type one S100 can be highly expressed and other neighboring S100 gene may be expressed at low level or unexpressed [4].
High and moderate expression level of S100A2 protein (also known as S100L or CaN19) was first observed in lung, kidney, liver, heart, and skeletal muscle with low levels detected in brain and intestine [5]. While S100A2 is expressed in many normal tissues, its aberrant expression in a number of tumor tissues has been reported [3, 6].The expression level of different S100 gene family members in cancer tissues seems to be regulated by epigenetic mechanisms. Among proteins which expression is effected by promoter methylation are S100A4, S100A6, S100A10, S100P which were shown to aberrantly hyper- or hypomethylated in breast cancer, colon, pancreas, prostate, gastric and endometrial cancer, among others [4]. S100A2 appears to be gene particularly targeted by hypermetylation. In breast and lung cancers, treatment of cell lines with the demethylating agent 5-aza-2’-deoxycytidine led to the re-expression of S100A2 mRNA, indicating that the lack of S100A2 expression may be at least partially associated with aberrant methylation of the promoter region [7, 8, 9]. In addition, S100A2 appears to positively regulate p53 transcriptional activity, while S100A4 expression strongly inversely correlates with p53 expression [10, 11]. Such evidence has led to the belief that S100A2 may be candidate tumor suppressor gene.
While the methylation status of S100A2 has been examined extensively in breast, lung, and prostate cancers, it has not been investigated in other cancers. In this study, we examined the status of 5’CpG island methylation of S100A2 in head&neck and bladder cancer. We show that loss of S100A2 expression in head and neck and bladder cancer cell lines is associated with the methylation of CpG islands and can be restored with 5-aza-2’-deoxycytidine treatment. Furthermore, we demonstrate high frequency of aberrant methylation of S100A2 in primary head&neck and bladder tumor samples.
RESULTS
S100A2 gene is silenced by hypermethylation in cancer cell lines
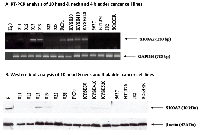
Using RT-PCR and Western blotting, we analyzed the expression patterns of S100A2 in 14 established head&neck and bladder cancer cell lines (Figure 1A and 1B). S100A2 expression was absent in four head and neck cancer cell lines (011, 012, 022, and 028) and all four bladder cancer cell lines (5637, HT1376, J82, and SCaBER). It was expressed in the other six head and neck cancer cell lines (013, 019, Fadu, KYSE30, KYSE410, and KYSE520).
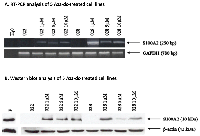
Thereafter, we performed pharmacological unmasking to verify if treatment with DNA methylation inhibitor 5-aza-2’-deoxycytidine (5Aza-dC) can lead to S100A2 re-expression. Six cell lines which did not express S100A2 (022, 028, 5637, HT1376, J82, and SCaBER) were treated with 5Aza-dC. In all of the treated cell lines, treatment with 5Aza-dC resulted in a robust re-expression of S100A2. Representative results for the head&neck cancer cell lines 022 and 028 are shown in Figure 2.
Then, we investigated the S100A2 promoter methylation status in all cell lines using bisulfite sequencing. Hypermethylation of the S100A2 promoter was found in the eight cancer cell lines which were previously found not to express S100A2 (011, 012, 022, 028, 5637, HT1376, J82, and SCaBER). As expected, no hypermethylation was detected in the cell lines that expressed S100A2. Quantitative methylation-specific PCR (QMSP) was then performed to determine more specifically the degree of promoter region methylation. The QMSP results were consistent with our bisulfite sequencing results; higher methylation values were detected in 8 cell lines (011, 012, 022, 028, 5637, HT1376, J82, and SCaBER), all of which had no S100A2 expression in RT-PCR or Western blotting analysis, whereas the head&neck cancer cell lines 013, 019, Fadu, KYSE30, KYSE410, and KYSE520, which express S100A2, were unmethylated in this promoter region. Incidence of promoter hypermethlation as observed in QMSP is summarized in Table 1.
S100A2 hypermethylation in primary head&neck and bladder tumor tissue samples.
To determine whether S100A2 promoter methylation was limited to cultured head&neck and bladder cancer cell lines, we examined the promoter methylation of S100A2 in primary head&neck and bladder cancers. Using QMSP, we examined the methylation status of S100A2 in 31 head and neck and 31 bladder tumor tissue samples as well as in 15 normal tissue samples from the head and neck and bladder. The frequency of methylation was significantly higher in the primary tumor samples as compared to the normal tissue samples (Table 1). 90% (28 of 31) of primary tumor samples from the head and neck demonstrated methylation while 11% (1 of 9) of normal head and neck tissues were methylated (p<0.01). In the bladder, 80% (25 of 31) of the primary tumor samples showed methylation while 33% (2 of 6) of the normal bladder tissues were methylated (p=0.017).
We correlated DNA hypermethylation of S100A2 in cancer samples with clinical and histopathological variables to determine whether these alterations were associated with any particular phenotype. In bladder cancer, the frequency of methylation was found to be significantly higher as the tumor grade increased (p<0.01). In the primary bladder cancer samples, methylation occurred in 33% of grade I (1 of 3) and grade II (1 of 3) urothelial cell carcinomas, whereas methylation occurred in 85% (11 of 13) grade III carcinomas. These data suggest that the loss of S100A2 expression may be an important event during the cancer progression. Aberrant S100A2 methylation in primary head&neck and bladder tumors had no correlation with patient demographic data, including age and gender, histological subtype, and staging of the tumor (data not shown).
S100A2 methylation in DNA isolated from urine in bladder cancer patients
To investigate whether S100A2 methylation could be potentially utilized for detection of bladder cancer, we investigated S100A2 methylation status in DNA isolated from urine samples. High frequency of methylation was observed in urine samples from cases in comparison with controls (96%vs. 48%; p<0.01, Chi-square test) (data not shown).
DISCUSSION
We have found aberrant methylation involving the promoter regions of S100A2 in different histologies of head&neck and bladder cancer. In cancer cell lines, DNA promoter hypermethylation correlated with loss of gene expression and could be efficiently restored with the de-methylation agent, 5-aza-2’-deoxycytidine. In addition, our findings of significantly high frequency of S100A2 methylation in primary head&neck and bladder cancer support the fact that epigenetic silencing of this gene may be a tumor-specific event. This result fits the paradigm that has now been widely documented in many malignancies of a reciprocal relationship between the density of methylated cytosine residues in the 5’ region of some gene promoters and the transcriptional activity of that gene. We also found S100A2 methylatation cases in normal head&neck and bladder tissue in 1 of 9 head&neck and 2 of 6 normal bladder tissues. At present we have no clear explanation of S100A2 promoter methylation in cases of normal head&neck and bladder tissue. Environmental factors or aging process may be responsible for this kind of alterations which may be a very early stage of cellular transformation. It should be noted that S100A2 promoter hypermethylation has previously been demonstrated in prostate cancer, but considerable levels of methylation were also present in some non-malignant prostate tissues [12]. Expression profiles of S100A2 indicate that it may be a candidate tumor suppressor gene [12, 13]. The role of S100A2 as a tumor suppressor is supported by study of BRCA1 mutant and basal-like breast cancer cell lines, in which S100A2 exogenous expression resulted in growth inhibition, while siRNA knockdown enhanced proliferation [14]. S100A2 transfection in melanoma cells revealed its anti-proliferative activity [15], in gastric cancer cells it seems to decrease cell invasiveness [16], and similarly in head and neck squamous cell carcinoma S100A2 reduces proliferation, cell motility and cancer cell invasion [17].
Additionally, aberrant S100A2 expression in cancer has been demonstrated in many studies. In support of antionconcogenic role of S100A2, the expression level of this gene shown to be down-regulated in breast cancer, melanoma, prostate cancer, non-small cell lung cancer, esophageal squamous cell carcinoma and gastric carcinoma [3, 7, 8, 12, 16, 18]. However studies are not consistent, as other reports show considerable levels of S100A2 mRNA and/or protein in non-small cell lung cancer [19], esophageal squamous cell carcinoma and Barrett’s adenocarcinoma [20, 21], and gastric cancer [22]. Moreover, other studies identified S100A2 up-regulation in a number of other malignancies, such as pancreas adenocarcinoma [23] and ovarian cancer [24], among others. Data from these studies put putative S100A2 antitumor role in question. It seems that its anti-proliferative role as a tumor suppressor may depend on particular cell and biological context and it needs to be further elucidated.
Potentially important role of S100A2 in carcinogenesis has been suggested. While few studies describing genetic alterations in S100A2 gene in cancer exist [25] , epigenetic mechanisms could also account for disturbance of S100A2 function. In support of this, S100A2 transcription repression in breast, lung, and prostate cancer cells and cell lines was shown to be mediated at least in part by site-specific methylation in the promoter region, similarly to a number of known and putative tumor suppressor genes [7, 9]. This report furthers these previous studies by demonstrating promoter region hypermethylation in head and neck and bladder cancer cell lines and primary cancer tissues. Our data show that S100A2 expression is silenced in many cancer cell lines and can be restored by 5-Aza-dC treatment.
Interestingly, in prostate cancer the frequency of methylation was significantly higher in grade III tumors as compared to grade I and II tumors. This finding confirmed two previous reports on prostate adenomacarcinoma showing that loss of S100A2 expression correlated with increasing tumor grade, suggesting that the loss of expression may be an important event in tumor progression [26]. Similarly, gradual loss of S100A2 expression was found from gastritis, through intestinal metaplasia and dysplasia to gastric cancer [27]. In head and neck squamous cell carcinoma, S100A2 expression was noted in some primary tumors, lymph node metastasis showed reduction in staining [28]. One the other hand, contrary to the results on differential S100A2 expression in different grades of prostate adenocarcinoma, no difference in methylation status of S100A2 was found between high-grade prostate intraepithelial neoplasia and benign prostate hyperplasia, in all of which observed methylation levels were consistently high [12]. This may suggest that not only methylation status is responsible for differential S100A2 expression in different stages of prostate malignancy.
While our report provides a thorough examination of the expression of S100A2 in head&neck and bladder, it should be noted that previous reports on the expression of S100A2 in head&neck cancer have been contradictory. Studies found that the expression of S100A2 was decreased in early-stage oral cancer cells and recurrent nasopharyngeal cancer [29, 30], however other investigators suggest S100A2 upregulation in HNSCC [31, 32]. Studies of head and neck squamous cell carcinoma show variable levels of S100A2 in different cell lines [33]. Another study found this gene to be expressed in more than 95% of low-grade tumors and 51% of high-grade tumors of laryngeal squamous cell carcinoma, however S100A2 negative cases were typically anaplastic non-keratinizing tumors that generally bear more malignant characteristics [34]. A study involving 424 normal and tumor tissues of various origin showed that while in normal non-epithelial tissues have low level of S100A2 expression, in normal epithelial tissue its expression is present but decreases in tumors of epithelial origin concurrently with loss of keratin K14. This loss was more pronounced in glandular than squamous epithelial tissues [35]. This is consistent with data showing that S100A2 is strongly expressed in epithelial basal cells and epithelial tumors of the skin [6, 36], and it was suggested that S100A2 may play role in differentiation towards keratinocyte phenotype [37]. Furthermore, in a previous report, S100A2 overexpression in squamous and basal cell carcinoma was often found to be associated with hyperplastic peri-lesional skin, and S100A2 presence in this cases may reflect regenerative squamous differentiation [38]. Therefore, it seems that loss of S100A2 might be an important event in tumor progression towards more malignant cell phenotype in head and neck cancer, but the decrease in its level may be also attributed to loss of epithelial phenotype.
As it was shown by many, tumor derived genetic disturbances can be detected in body fluids and used as cancer markers. Here, we investigated if hypermethylation of S100A2 can be detected in urine samples of bladder cancer cases. Results presented here showed that frequency of hypermethylation of S100A2 is significantly higher in urine samples from bladder tumor cases than in healthy subjects (96% vs 48% respectively). This may suggest that S100A2 promoter methylation is a common event in bladder cancer and it can be detected in urine. However, due to the limited number of urine samples analyzed, we were not able to determine empirical cut off value to determine optimal sensitivity and specificity. Further study using large cohort of samples is needed to determine the feasibility S100A2 promoter methylation as a screening marker in urine. Furthermore, analytical sensitivity needs to be determined before testing extended urine samples. In a recent study, while noting presence of S100A2 methylation in head and neck squamous cell cancer tissue, very limited hypermethylation of S100A2 was detected in saliva and serum in this malignancy [39]. Therefore, application of S100A2 methylation for detection of head&neck and bladder cancers requires further evaluation.
In summary, we have demonstrated aberrant S100A2 gene methylation in human head&neck and bladder cancer and most importantly promoter methylation of S100A2 is inversely associated with gene expression. Because S100A2 gene methylation is significantly more frequent in higher grade tumors, it may have a use as a marker of tumor progression.
MATERIALS AND METHODS
Cell lines, primary tumor and urine samples
The head and neck tumor cell lines Fadu, and bladder cancer cell lines 5637, SCaBER, HT1376, and J82 were obtained from ATCC (Manassas, VA). The head and neck cancer cell lines 011, 012, 013, 019, 022, and 028 were established in the Department of Otolaryngology – Head and Neck Surgery at The Johns Hopkins University. Head&neck cancer cell lines KYSE30, KYSE410, and KYSE520 were kindly provided by Dr. Shimada in the Department of Surgery, Kyoto University. 31 head and neck primary tumors and 31 bladder primary tumors from the Johns Hopkins Hospital were selected to determine the S100A2 methylation frequency in primary tumors. Samples were stored frozen in-80 ̊C. 50 ml of voided urine were collected from 23 cases prior to definitive surgery at Johns Hopkins University School of Medicine and from 24 control patients with no history of genitourinary malignancy. Urine samples were centrifuged at 3000 X g for 10 minutes and the pallet was washed twice with phosphate-buffered saline (PBS) and frozen at -80 ̊C. Approval for the study was obtained from JHU Institutional Review Board.
Bisulfite treatment and QMSP
Genomic DNA was isolated from cell lines, tissues and urine samples by digestion with proteinase K (0.5 mg/ml) in 1% SDS, Tris (1M, pH 8.8), EDTA (0.5M, pH 8.0), and NaCl (5M) overnight at 48ºC followed by phenol/chloroform extraction and ethanol precipitation. Sodium bisulfite conversion of unmethylated cytosine residues to uracil of genomic DNA was performed. 2 μg of genomic DNA in 20 μl of H2 O containing 5 μg of salmon sperm DNA were denatured by incubation with 0.3M NaOH at 50ºC for 20 min. DNA was then incubated at 70ºC for 3 h in a 500 μl reaction mixture containing 2.5M sodium metabisulfite and 0.125M hydroquinone (pH 5.0). Treated DNA was purified with the Wizard DNA purification system according to the manufacturer instructions (Promega Corp., Madison, WI), and finally the bisulfite-modified DNA was resuspended in LoTE (2.5mM EDTA, 10mM Tris-HCL).
Bisulfite-modified DNA was used as a template for fluorescence-based real-time PCR (Taqman) as previously described [11]. Briefly, primers and probes were designed to specifically amplify the bisulfite-converted promoter of the gene of interest. The primer and probe sequences used for detection of target gene (S100A2, GenBank Accession #Y07755) and internal reference gene (β-actin, ACTB) are provided in Supplement Table 1.
The ratio of QPCR values of the gene of interest to β-actin was used as a measure of relative methylation level (target gene/β-actin × 1000). QPCR was performed in 20 µl reaction volumes containing 600nM of each primer; 200nM of probe; 0.75 units of platinum Taq polymerase (Invitrogen); 200µM of dATP, dCTP, dGTP, and dTTP each; 16.6mM ammonium sulfate; 67mM Trizma; 6.7mM MgCl2; 10mM mercaptoethanol; and 0.1% DMSO. 3 µl of treated DNA solution were used in each reaction and amplifications were carried out in a 7900 Sequence detector (Perkin-Elmer Applied Biosystems). PCR plates consisted of patient samples and multiple water blanks, as well as positive and negative controls. Leukocytes from a healthy individual were methylated in vitro with excess SssI methyltransferase (New England Biolabs Inc., Beverly, MA) to generate completely methylated DNA and serial dilutions of this DNA were used to construct calibration curves.
Bisulfite sequencing
Bisulfite-treated DNA was subjected to PCR with primers flanking the targeted methylation-specific PCR regions. PCR products were then gel-purified and sequenced. Sequences of primers used for the amplification and sequencing are provided in Supplement Table 2.
5’-aza-2’-deoxycytidine treatment
022, 028, 5637, SCaBER, HT1376, and J82 were split to low density 48 h before treatment in 6-well plates. Cells were then treated for 3 days with 1, 5, and 10μM of 5’-aza-2’-deoxycytidine (Sigma) or were mock-treated with same volumes of 1X PBS. After the treatment, proteins and RNA were harvested.
RNA isolation and RT-PCR
Total RNA was isolated using Qiazol reagent (Qiagen). Agarose gel electrophoresis at 1% and spectrophotometric analysis were used to assess RNA quality. First-stand cDNA was synthesized using random primers and M-MLV reverse transcriptase according to manufacturer’s protocol with little modification (Invitrogen). cDNA was subjected to PCR using primers spanning exons 2 and 3 of S100A2 (GenBank Accession number Y07755). Sequences of primers used for RT-PCR are provided in Supplement Table 3. As an internal control, Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified to ensure cDNA quality and quantity for each RT-PCR. Final PCR products were resolved on a 2% TBE agarose gel and visualized.
Western blot analysis
PBS-washed cells were lysed by sonication in ice-cold RIPA buffer (50mM Tris-HCl, pH 7.4, 150mM NaCl, 1mM PMSF, 1mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS). Protein concentrations were determined by the Bio-Rad DC protein assay (Bio-Rad). Equal amounts of protein were subjected to 16% SDS-polyacrylamide gel electrophoresis, and the proteins were transferred onto PVDF membranes. After blotting with 5% nonfat dry milk and 0.1% Tween-20 in 1X PBS, membranes were probed with mouse anti-S100L antibody (BD Transduction Laboratories) at a 1:1000 dilution in the blotting buffer for one hour at room temperature. After washing with 1X PBS and 0.1% Tween-20, the membranes were incubated with secondary antibody, HRP-conjugated anti-mouse antibody (Amersham), at a 1:5000 dilution in the blotting buffer. The membranes were again washed with 1X PBS and 0.1% Tween-20 and chemiluminescence system (Amersham) was used for protein detection. MDBK cell lysate provided by BD Transduction Laboratories was used as a positive control.
Statistical analysis
Statistical tests were performed with Chi-square test using Statistica 10 software (Statsoft).
FUNDING
This study was supported in part by the SPORE grant P50 CA96784-01 (to C.M.) and Cancer Research Grant by Pyung Ya Foundation (C.M.). The study was also supported by Flight Attendant Medical Research Institute Young Clinical Scientist Award, Career Development award from SPORE in Cervical Cancer Grants P50 CA098252 (M.H.) and 1R01CA163594-01 (D.S. and M.H.).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.

- 1. Gross SR, Sin CG, Barraclough R, Rudland PS. Joining S100 proteins and migration: for better or for worse, in sickness and in health. Cell Mol Life Sci 2014; 71: 1551-1579. https://doi.org/10.1007/s00018-013-1400-7. [PubMed].
- 2. Ilg EC, Schafer BW, Heizmann CW. Expression pattern of S100 calcium-binding proteins in human tumors. Int J Cancer 1996; 68: 325-332. https://doi.org/10.1002/(SICI)1097-0215(19961104)68:3<325::AID-IJC10>3.0.CO;2-7. [PubMed].
- 3. Leclerc E, Heizmann CW, Vetter SW. RAGE and S100 protein transcription levels are highly variable in human melanoma tumors and cells. Gen Physiol Biophys 2009; 28 Spec No Focus: F65-75.
- 4. Lesniak W. Epigenetic regulation of S100 protein expression. Clinical epigenetics 2011; 2: 77-83. https://doi.org/10.1007/s13148-011-0023-9. [PubMed].
- 5. Glenney JR, Jr., Kindy MS, Zokas L. Isolation of a new member of the S100 protein family: amino acid sequence, tissue, and subcellular distribution. J Cell Biol 1989; 108: 569-578. https://doi.org/10.1083/jcb.108.2.569. [PubMed].
- 6. Zhu L, Kohda F, Nakahara T, Chiba T, Tsuji G, Hachisuka J et al. Aberrant expression of S100A6 and matrix metalloproteinase 9, but not S100A2, S100A4, and S100A7, is associated with epidermal carcinogenesis. J Dermatol Sci 2013; 72: 311-319. https://doi.org/10.1016/j.jdermsci.2013.07.005. [PubMed].
- 7. Lee SW, Tomasetto C, Swisshelm K, Keyomarsi K, Sager R. Down-regulation of a member of the S100 gene family in mammary carcinoma cells and reexpression by azadeoxycytidine treatment. Proceedings of the National Academy of Sciences of the United States of America 1992; 89: 2504-2508. https://doi.org/10.1073/pnas.89.6.2504. [PubMed].
- 8. Feng G, Xu X, Youssef EM, Lotan R. Diminished expression of S100A2, a putative tumor suppressor, at early stage of human lung carcinogenesis. Cancer research 2001; 61: 7999-8004.
- 9. Wicki R, Franz C, Scholl FA, Heizmann CW, Schafer BW. Repression of the candidate tumor suppressor gene S100A2 in breast cancer is mediated by site-specific hypermethylation. Cell calcium 1997; 22: 243-254. https://doi.org/10.1016/s0143-4160(97)90063-4. [PubMed].
- 10. Mueller A, Schafer BW, Ferrari S, Weibel M, Makek M, Hochli M et al. The calcium-binding protein S100A2 interacts with p53 and modulates its transcriptional activity. J Biol Chem 2005; 280: 29186-29193. https://doi.org/10.1074/jbc.M505000200. [PubMed].
- 11. Matsubara D, Niki T, Ishikawa S, Goto A, Ohara E, Yokomizo T et al. Differential expression of S100A2 and S100A4 in lung adenocarcinomas: clinicopathological significance, relationship to p53 and identification of their target genes. Cancer Sci 2005; 96: 844-857.
- 12. Rehman I, Cross SS, Catto JW, Leiblich A, Mukherjee A, Azzouzi AR et al. Promoter hyper-methylation of calcium binding proteins S100A6 and S100A2 in human prostate cancer. Prostate 2005; 65: 322-330. https://doi.org/10.1002/pros.20302. [PubMed].
- 13. Gupta S, Hussain T, MacLennan GT, Fu P, Patel J, Mukhtar H. Differential expression of S100A2 and S100A4 during progression of human prostate adenocarcinoma. J Clin Oncol 2003; 21: 106-112. https://doi.org/10.1200/JCO.2003.03.024. [PubMed].
- 14. Buckley NE, D’Costa Z, Kaminska M, Mullan PB. S100A2 is a BRCA1/p63 coregulated tumour suppressor gene with roles in the regulation of mutant p53 stability. Cell Death Dis 2014; 5: e1070. https://doi.org/10.1038/cddis.2014.31. [PubMed].
- 15. Foser S, Redwanz I, Ebeling M, Heizmann CW, Certa U. Interferon-alpha and transforming growth factor-beta co-induce growth inhibition of human tumor cells. Cell Mol Life Sci 2006; 63: 2387-2396. https://doi.org/10.1007/s00018-006-6256-7. [PubMed].
- 16. Liu YF, Liu QQ, Wang X, Luo CH. Clinical significance of S100A2 expression in gastric cancer. Tumour Biol 2014; 35: 3731-3741. https://doi.org/10.1007/s13277-013-1495-3. [PubMed].
- 17. Tsai WC, Tsai ST, Jin YT, Wu LW. Cyclooxygenase-2 is involved in S100A2-mediated tumor suppression in squamous cell carcinoma. Mol Cancer Res 2006; 4: 539-547. https://doi.org/10.1158/1541-7786.MCR-05-0266. [PubMed].
- 18. Cao LY, Yin Y, Li H, Jiang Y, Zhang HF. Expression and clinical significance of S100A2 and p63 in esophageal carcinoma. World J Gastroenterol 2009; 15: 4183-4188. https://doi.org/10.3748/wjg.15.4183. [PubMed].
- 19. Bartling B, Rehbein G, Schmitt WD, Hofmann HS, Silber RE, Simm A. S100A2-S100P expression profile and diagnosis of non-small cell lung carcinoma: impairment by advanced tumour stages and neoadjuvant chemotherapy. Eur J Cancer 2007; 43: 1935-1943. https://doi.org/10.1016/j.ejca.2007.06.010. [PubMed].
- 20. Lee OJ, Hong SM, Razvi MH, Peng D, Powell SM, Smoklin M et al. Expression of calcium-binding proteins S100A2 and S100A4 in Barrett’s adenocarcinomas. Neoplasia 2006; 8: 843-850. https://doi.org/10.1593/neo.06481. [PubMed].
- 21. Imazawa M, Hibi K, Fujitake S, Kodera Y, Ito K, Akiyama S et al. S100A2 overexpression is frequently observed in esophageal squamous cell carcinoma. Anticancer Res 2005; 25: 1247-1250.
- 22. Liu J, Li X, Dong GL, Zhang HW, Chen DL, Du JJ et al. In silico analysis and verification of S100 gene expression in gastric cancer. BMC Cancer 2008; 8: 261. https://doi.org/10.1186/1471-2407-8-261. [PubMed].
- 23. Ohuchida K, Mizumoto K, Miyasaka Y, Yu J, Cui L, Yamaguchi H et al. Over-expression of S100A2 in pancreatic cancer correlates with progression and poor prognosis. J Pathol 2007; 213: 275-282. https://doi.org/10.1002/path.2250. [PubMed].
- 24. Santin AD, Zhan F, Bellone S, Palmieri M, Cane S, Bignotti E et al. Gene expression profiles in primary ovarian serous papillary tumors and normal ovarian epithelium: identification of candidate molecular markers for ovarian cancer diagnosis and therapy. Int J Cancer 2004; 112: 14-25.
- 25. Strazisar M, Rott T, Glavac D. Frequent polymorphic variations but rare tumour specific mutations of the S100A2 on 1q21 in non-small cell lung cancer. Lung Cancer 2009; 63: 354-359. https://doi.org/10.1016/j.lungcan.2008.06.005. [PubMed].
- 26. Kwon YW, Chang IH, Kim KD, Kim YS, Myung SC, Kim MK et al. Significance of S100A2 and S100A4 Expression in the Progression of Prostate Adenocarcinoma. Korean J Urol 2010; 51: 456-462. https://doi.org/10.4111/kju.2010.51.7.456. [PubMed].
- 27. Zhao Y, Zhang TB, Wang Q. Clinical significance of altered S100A2 expression in gastric cancer. Oncology reports 2013; 29: 1556-1562. https://doi.org/10.3892/or.2013.2236. [PubMed].
- 28. Zhang X, Hunt JL, Shin DM, Chen ZG. Down-regulation of S100A2 in lymph node metastases of head and neck cancer. Head Neck 2007; 29: 236-243. https://doi.org/10.1002/hed.20511. [PubMed].
- 29. Tsai ST, Jin YT, Tsai WC, Wang ST, Lin YC, Chang MT et al. S100A2, a potential marker for early recurrence in early-stage oral cancer. Oral Oncol 2005; 41: 349-357. https://doi.org/10.1016/j.oraloncology.2004.09.006. [PubMed].
- 30. Huang Z, Li W, Lin S, Fang X, Zhang C, Liao Z. Identification of novel tumor suppressor genes down-regulated in recurrent nasopharyngeal cancer by DNA microarray. Indian J Otolaryngol Head Neck Surg 2014; 66: 120-125. https://doi.org/10.1007/s12070-011-0359-7. [PubMed].
- 31. Dysvik B, Vasstrand EN, Lovlie R, Elgindi OA, Kross KW, Aarstad HJ et al. Gene expression profiles of head and neck carcinomas from Sudanese and Norwegian patients reveal common biological pathways regardless of race and lifestyle. Clinical cancer research : an official journal of the American Association for Cancer Research 2006; 12: 1109-1120.
- 32. Tyszkiewicz T, Jarzab M, Szymczyk C, Kowal M, Krajewska J, Jaworska M et al. Epidermal differentiation complex (locus 1q21) gene expression in head and neck cancer and normal mucosa. Folia Histochem Cytobiol 2014; 52: 79-89. https://doi.org/10.5603/FHC.2014.0018. [PubMed].
- 33. Nagy N, Brenner C, Markadieu N, Chaboteaux C, Camby I, Schafer BW et al. S100A2, a putative tumor suppressor gene, regulates in vitro squamous cell carcinoma migration. Lab Invest 2001; 81: 599-612. https://doi.org/10.1038/labinvest.3780269. [PubMed].
- 34. Lauriola L, Michetti F, Maggiano N, Galli J, Cadoni G, Schafer BW et al. Prognostic significance of the Ca(2+) binding protein S100A2 in laryngeal squamous-cell carcinoma. Int J Cancer 2000; 89: 345-349. https://doi.org/10.1002/1097-0215(20000720)89:4<345::aid-ijc5>3.0.co;2-t. [PubMed].
- 35. Nagy N, Hoyaux D, Gielen I, Schafer BW, Pochet R, Heizmann CW et al. The Ca2+-binding S100A2 protein is differentially expressed in epithelial tissue of glandular or squamous origin. Histology and histopathology 2002; 17: 123-130. https://doi.org/10.14670/HH-17.123. [PubMed].
- 36. Park HR, Min SK. Expression of S100A2 and S100B proteins in epithelial tumors of the skin. J Cutan Pathol 2003; 30: 373-378. https://doi.org/10.1034/j.1600-0560.2003.00081.x. [PubMed].
- 37. Lapi E, Iovino A, Fontemaggi G, Soliera AR, Iacovelli S, Sacchi A et al. S100A2 gene is a direct transcriptional target of p53 homologues during keratinocyte differentiation. Oncogene 2006; 25: 3628-3637.
- 38. Xia L, Stoll SW, Liebert M, Ethier SP, Carey T, Esclamado R et al. CaN19 expression in benign and malignant hyperplasias of the skin and oral mucosa: evidence for a role in regenerative differentiation. Cancer research 1997; 57: 3055-3062.
- 39. Carvalho AL, Jeronimo C, Kim MM, Henrique R, Zhang Z, Hoque MO et al. Evaluation of promoter hypermethylation detection in body fluids as a screening/diagnosis tool for head and neck squamous cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research 2008; 14: 97-107.
- 40. Hoque MO, Begum S, Topaloglu O, Jeronimo C, Mambo E, Westra WH et al. Quantitative detection of promoter hypermethylation of multiple genes in the tumor, urine, and serum DNA of patients with renal cancer. Cancer research 2004; 64: 5511-5517. https://doi.org/10.1158/0008-5472.CAN-04-0799. [PubMed].
Last Modified: 2016-06-04 00:50:59 EDT
