Oncoscience
IPIAD- an augmentation regimen added to standard treatment of pancreatic ductal adenocarcinoma using already-marketed repurposed drugs irbesartan, pyrimethamine, itraconazole, azithromycin, and dapsone
Richard E. Kast1
1IIAIGC Study Center, Burlington, VT 05401, USA
Correspondence to: Richard E. Kast, email: [email protected]
Keywords: gemcitabine; multidrug; paclitaxel; pancreatic ductal adenocarcinoma; repurposed
Received: October 30, 2023
Accepted: January 01, 2024
Published: February 07, 2024
Copyright: © 2024 Kast. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
This short note presents the data and rationale for adding five generic non-oncology drugs from general medical practice to gemcitabine, nab-paclitaxel, a current standard cytotoxic chemotherapy of pancreatic ductal adenocarcinoma. The regimen, called IPIAD, uses an angiotensin receptor blocker (ARB) irbesartan indicated for treating hypertension, an old antimicrobial drug pyrimethamine indicated for treating toxoplasmosis or malaria, an old antifungal drug itraconazole, an old broad spectrum antibiotic azithromycin and an old antibiotic dapsone. In reviewing selected growth driving systems active in pancreatic ductal adenocarcinoma then comparing these with detailed data on ancillary attributes of the IPIAD drugs, one can predict clinical benefit and slowing growth of pancreatic ductal adenocarcinoma by this augmentation regimen.
INTRODUCTION
This paper presents the rationale for adding five already approved and marketed generic drugs from general medical practice to the current standard current first line chemotherapy for pancreatic ductal adenocarcinoma (PDAC). This adjuvant regimen, called by its acronym, IPIAD, uses an old angiotensin receptor blocker (ARB) irbesartan, an old antimicrobial drug pyrimethamine, an old antifungal drug itraconazole, an old antibiotic azithromycin, and an old antibiotic dapsone.
PDAC is a highly desmoplastic tumor where nonmalignant tumor stroma plays a more active symbiotic role in growth of the malignant cells than is seen in most other cancers. The nonmalignant cells of PDAC grow in a trophic and immunosuppressive tumor environment that has neutrophils, macrophages, fibroblasts, and a desmoplastic stroma rich in hyaluronan [1]. This stroma requires a separate treatment regimen from the malignant PDAC cells. Survival rates at 5 years are low. At presentation, half of all PDAC have already metastasized. For unclear reasons, incidence of PDAC has been steadily increasing at 0.5 to 1% per year [1].
The IPIAD regimen was designed specifically to improve standard current chemotherapies for metastatic PDAC particularly in cases where the benefits of resection is questionable. Pancreatectomy and even oligometastases resection can be offered to those achieving tumor reduction with chemotherapy. IPIAD, by inhibiting aspects of growth drive in PDAC as outlined here, was constructed to help current standard gemcitabine, nab-paclitaxel achieve this bulk reduction with consequent survival benefits. May reduce the local mass size, promote tumor downstaging, and increase the likelihood of resection. Patients presenting with inoperable metastatic PDAC who experience a major response after neoadjuvant treatment can occasionally be restaged, have an increased survival, and some may then have tumors small enough to resect [1–3].
The IPIAD drugs were chosen by searching for potentially constructive intersections between the pathophysiologic growth drive mechanisms used by PDAC with the primary or ancillary physiological effects of common, low side effect medicines used in general medical practice (non-oncology drugs) that would interfere with them. The primary consideration in selecting drugs included in IPIAD was their having a low side effect risk and good safety when used in general medical practice.
This is the process that led to several repurposed multidrug regimens for adjunctive treatment of cancer, mainly glioblastoma, examples listed in Table 1 [4–9]. In these papers we outlined the reasons why a paradigm shift to multidrug regimens will be required to significantly stop a metastasized deadly cancer like glioblastoma or PDAC and why repurposed older drugs from general medicine should comprise many of those drugs. These reasons are summarized in Table 2.
The recent pilot clinical study of one such multidrug regimen for glioblastoma, CUSP9v3, showed that ten drugs to treat this cancer can be given daily over many years with benefit and without problems if close patient follow up is in place and a low-risk drug mix was carefully chosen [4].
As of this writing in 2023, widely metastatic PDAC is usually incurable [10]. Small PDACs, caught and removed early, before metastasis, have a good prognosis, a 5 yr. Overall survival of >80% [11]. Thus current efforts are aimed at reducing the extent of PDAC tumor burden with pre-surgical chemotherapy and/or irradiation [12, 13].
Current chemotherapies for PDAC commonly use either a) gemcitabine, nab-paclitaxel or b) FOLFIRINOX (folinic acid, fluorouracil, irinotecan, oxaliplatin) [14]. IPIAD is designed to contribute to tumor mass downsizing, to be used alongside gemcitabine, nab-paclitaxel.
Below, the attributes of irbesartan, pyrimethamine, itraconazole, azithromycin, and dapsone are reviewed with focus on how these attributes interact with several established PDAC growth driving systems in a way that is predicted to meet our goals of tumor mass downsizing. Four of the IPIAD drugs, pyrimethamine, itraconazole, azithromycin, and dapsone have been discussed in the context of adjunctive treatment of glioblastoma, shown in Table 1.
Irbesartan
The drug
Irbesartan is an ARB that binds to the angiotensin II receptor 1, preventing receptor activation by angiotensin II. Irbesartan is commonly used to treat hypertension [15, 16]. Side effects are minimal and usually well tolerated. Angiotensin converting enzyme inhibitors (ACEi) are a related older class of medicine for treating hypertension by reducing conversion of angiotensin I to angiotensin II.
Intersection with PDAC
A strong rationale for adding irbesartan to gemcitabine, nab-paclitaxel are four epidemiological studies:
- The empirical data of Zhou et al. showed longer median survival in advanced PDAC in people who took irbesartan, ~15 months, alongside of gemcitabine, nab-paclitaxel, compared to those on gemcitabine, nab-paclitaxel alone, ~10 months [17]. That comparative study was done on the basis of preclinical study showing that PDAC with low expression of c-Jun responded better to gemcitabine compared to PDACs with high c-Jun expression [17] - that plus demonstrations that irbesartan lowered PDAC expression of c-Jun. It is noteworthy in this context of c-Jun and gemcitabine, that a 2015 study showed that PDAC survival prolongation by ARB use was particularly seen in those on a gemcitabine containing regimen [18, 19].
- A 2023 study using a different cohort of 700 PDAC cases showed an association of angiotensin II signaling inhibition by ARBs or ACEi and longer survival compared to those not using an ARB or ACEi [20].
- The epidemiological study of Keith et al. looking at 8,000 PDAC cases of all stages. They found a reduction in PDAC mortality in ARB users and some but lesser benefit from ACEi use [21].
- Cerullo et al. studied records of 4299 PDAC cases who had resection for localized PDAC. Those on an ARB had a 24% reduced risk of death at 5 years [22].
A 2013 clinical trial in advanced metastatic PDAC using an ARB similar to irbesartan, candesartan 16 mg/day, plus standard gemcitabine gave a progression free survival (PFS) and overall survival that the authors considered similar to historical controls [23]. However, PFS was 3.5 months in those receiving 8 mg candesartan/day and 4.6 months in those receiving 16 mg/day [23]. Does this mean using the maximum recommended dose of candesartan, 32 mg/day, will give incremental benefit? Cf. the orthopedics aphorism “if a little force doesn’t work, maybe more force will”. Since PDAC tumors synthesize angiotensin II without use of ACE [24] this may account for the stronger PDAC growth inhibition seen with ARBs compared to ACEi.
Hypotension is a risk with any ARB use but can be frequently addressed in normotensives by some volume expansion with intake of a liter of salty tomato juice like V-8 juice™ or dose reduction.
Multiple animal studies showing ARB use inhibited PDAC growth preceded clinical ARB studies in PDAC [25–28].
One epidemiological study failed to find an association between ARB use and survival in PDAC [29].
Recognition that angiotensin II signaling forms one of the many overactive signaling systems active in glioblastoma led to inclusion of an angiotensin converting enzyme inhibitor in the CUSP9v3 regimen [4]. Many organs, including pancreas, have endogenous renin-angiotensin systems functioning independently from the renal-hepatic-lung system associated with hypertension [30]. The renin-angiotensin signaling system is often found deranged or hijacked to promote malignant growth across the common human cancers [31].
A further and important reason to add an ARB like irbesartan to PDAC treatment should be noted. The presence of sarcopenia is common and lowers survival duration in PDAC [32, 33]. Angiotensin II signaling at angiotensin II receptor 1, the receptor that ARBs block, contributes to sarcopenia across several diseases including heart failure, Alzheimer’s disease, and the common cancers [34, 35]. Several sartan class ARBs lowered muscle loss in patients with cardiovascular disease [36], in hemodialysis patients [37], and in old mice [38].
Pyrimethamine
The drug
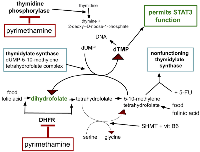
Pyrimethamine is an old generic antibiotic used today mainly in treatment or prophylaxis against Toxoplasma gondii, Pneumocystis jirovecii, Plasmodia and other protists. Pyrimethamine is one of several antibiotics that are based on dihydrofolate reductase (DHFR) inhibition. It is a lipophilic DHFR similar to hydrophilic methotrexate. Pyrimethamine also inhibits thymidine phosphorylase, methotrexate does not. How these inhibitions fit into folate metabolism is depicted in Figure 1.
Intersection with PDAC
Pyrimethamine has inhibitory effects on four growth drive elements of PDAC - (1) DHFR, (2) myeloid derived suppressor cells (MDSC), (3) STAT3, and (4) thymidine phosphorylase.
DHFR
DHFR is essential for normal functioning of the folate cycle. Pyrimethamine’s antibiotic activity in treating malaria, toxoplasmosis, pneumocystis, etc is based on a preferential inhibition of microbial DHFR compared to human but it does also inhibit human DHFR [39, 40]. Methotrexate is a standard DHFR inhibitor used in several cancer chemotherapies [41–43].
Pyrimethamine’s Ki = 38 nM at DHFR, is comparable to that of the more commonly used DHFR inhibitor methotrexate, Ki = 2.3 nM or the DHFR natural substrate folinic acid Ki = 320 nM, and folic acid Ki = 830 nM [39, 40]. For methotrexate to be active, it must be retained within the cell, which occurs when it becomes polyglutamated. Pyrimethamine does not require polyglutamination to be retained within cells. Pyrimethamine treatment at higher doses can give a reversible bone marrow suppression, making periodic blood monitoring advisable [44, 45].
In an acute myelogenous leukemia model, pyrimethamine was more effective in inhibiting growth than was methotrexate. In vitro proliferation was reduced 2.5 fold at 0.1 µM and 12.7 fold at 0.5 µM [46]. Several patients with polycythaemia vera and with essential thrombocythemia were successfully controlled with pyrimethamine, reported in 1987 [47].
Steady state pyrimethamine plasma level is ~1 μM at about 2–3 weeks following a dose of 12.5 mg/day, compared to ~6 μM with 50 mg/day [48]. Pyrimethamine has exhibited significant in vitro cytotoxicity to chronic lymphocytic leukemia cells within this dose range while showing no cytotoxicity to mixed leukocytes from healthy normals [48]. It is unclear why early reports in the 1970s of successful pyrimethamine treatment (2 mg/kg/day for 7 days) of meningeal recurrence of acute lymphoblastic leukemia in children have not been followed up [49].
MDSC and STAT3
Morphologically MDSC appear on H&E as neutrophils or monocytes. Neutrophil MDSC are CD15+ CD14− CD33dim HLA-DRneg. Monocyte MDSC are CD15− CD14 + CD33pos HLA-DRneg [50]. Although MDSC function is important in immune response moderation and normal wound repair, MDSC exert both immune response inhibiting and tumor growth trophic effects in the common cancers, including in PDAC [51–53].
STAT3 is a ~800 amino acid, 92 kDa transcription factor resident in an inactive form in cytoplasm under basal conditions. It is a member of a family of seven closely related transcription factors that are essential for normal cells’ function but often elevated and overactive in the common cancers, including in PDAC [54–56].
STAT3 is a signaling hub protein, a meeting point of many cellular signaling pathways, activated (phosphorylated) via many different inputs from these pathways. Several different cytokines acting on the outer cell membrane are transduced through the STAT3 signalling hub. Once activated (phosphorylated), P-STAT3 translocates to the nucleus as a dimer, where it binds to 9 base pair regions in the promoters of target genes to regulate transcription. In addition to regulating genes controlling proliferation, survival, and pluripotency, increase in P-STAT3 activity is a feature of inflammation [57–59]. P-STAT3 signaling plays a central role in MDSC survival and the production of immunoinhibiting arginase in MDSCs [60]. Elevation of P-STAT3 function drives increasing MDSC in PDAC [61–64].
MDSC synthesize and secrete arginase with consequent local arginine depletion inhibits T cell function [51, 65, 66]. Local arginase is just one of several T cell suppressing factors active in PDAC [53, 67, 68].
STAT3 is activated in PDAC intratumoral MDSC. Inhibiting STAT3 function reduces MDSC’s immune suppressing activity [69–72]. Inhibition of STAT3 with pyrimethamine in a murine breast cancer model resulted in decreased in vivo tumor growth and enhanced immune response [73]. Pyrimethamine similarly inhibited STAT3 and mesothelioma cell growth while having minimal effect on non-malignant pleural cell growth [74].
P-STAT3 activity is but one of the coalition of drivers increasing MDSC [75–77]. Inhibition of P-STAT3 function generally reduces the numbers and function of MDSC [78–80]. A study demonstrated a pyrimethamine mediated inhibition of STAT3 and proliferation of lung adenocarcinoma cells [81]. It has yet to be shown if the specific P-STAT3 function inhibition produced by pyrimethamine in clinical treatment of PDAC does this also or not.
Thymidine phosphorylase
Thymidine phosphorylase catalyzes phosphorylation of thymidine or deoxyuridine to respectively thymine or uracil and 2-deoxyribose 1-phosphate as in Figure 1. This is an essential step in thymidine recycling.
Thymidine phosphorylase activity is commonly increased in malignant tissue across the common cancers [82–84] and is specifically so in PDAC where increased thymidine phosphorylase levels correlated with shorter survival [85–89]. Thymidine phosphorylase is indeed a worthwhile target to inhibit in PDAC.
Itraconazole
The drug
Itraconazole is a commonly used antifungal drug used to treat both minor skin infections with Tinea, Candida, Malassezia spp., etc., or more serious invasive fungal disease. Itraconazole is one of the CUSP9v3 drugs used in glioblastoma treatment and is seeing increasing use as adjunct in other cancers’ chemotherapies [4, 90]. By blocking fungal ergosterol synthesis, itraconazole disrupts fungal cell wall integrity resulting in fungal cell death [91].
A major problem in interpreting clinical data on itraconazole in PDAC is its poor and erratic absorption. Itraconazole must be given with 200–300 ml of a low pH liquid - Coke™, orange juice, or 15 ml of vinegar 5% acetic acid in 200 ml water are examples. It has been common to prescribe itraconazole without mentioning this essential aid to itraconazole’s absorption.
Preclinical data
Older reviews outlined several ways itraconazole interferes with cancer growth [3, 92, 93].
A Bak-1 activation dependent apoptosis was identified in CFPAC-1 cells. These data suggested that itraconazole exhibited antiproliferative effects in PDAC by inducing apoptosis through Bak-1 activation. [94]. Itraconazole induced PDAC cells’ cytotoxicity in vitro that could be reversed by recombinant TGF-beta [95].
Hedgehog
Itraconazole inhibits Hedgehog signaling (Hh) [96–99]. Hh signaling is a core signalling element in normal organogenesis [100–103]. Excessive Hh signaling is an important growth driver in PDAC [104–107] as it is in many of the common deadly cancers [108–110].
Antimicrobial function
Since the pancreatic duct has direct communication with the duodenum at the major duodenal papilla, and PDAC usually starts a few cm distal to that, it is not surprising that PDAC tissue is not normally sterile [111]. Retrograde travel deep into the pancreatic tree would be easy and has been shown to occur. Bacterial DNA was detected in 76% of resected PDACs and in 15% of normal pancreases [112]. Akut et al. found PDAC tissue had ~3000-fold increased fungal DNA compared to normal pancreases [113]. Malassezia, Pseudoxanthomonas, Streptomyces, Saccharopolyspora and Bacillus clausii, Porphyromonas gingivalis and Fusobacterium nucleatum can be found within PDAC resected tissue [111–116].
Details of the relationship between microbial pancreas colonization and PDAC development have not been worked out in detail but the risk/benefit would favor use of itraconazole (and azithromycin, vide infra) on the basis of such colonization and the evidence that such colonization is, in fact, an element promoting PDAC growth.
Empirical
A 2022 study of Sawasaki et al. treated 81 PDAC cases with 400 mg itraconazole/day for four days every 14 day cycle. The authors felt this resulted in longer survival compared to historical controls but this report lacked important details, preventing further interpretation [117].
A similar study in 2015 reported 38 advanced PDAC cases given docetaxel, gemcitabine, and carboplatin one day every 14 days with itraconazole 400 mg/d on days −2 to +2 from that. The authors felt this resulted in longer survival compared to historical controls but this report too lacked details that would allow further interpretation [93].
Both these studies were seriously flawed and lacked a true control group. The dose of itraconazole in these studies was needlessly low and the ten days of no itraconazole between dose days not justified by the ferocity of the disease they aimed to treat or by the pharmacokinetics of itraconazole.
Since itraconazole disrupts function of mammalian focal adhesion kinase with consequent decreased cell motility and angiogenesis [118], plus Hh and the other growth elements itraconazole inhibits, it should be given at high doses (as tolerated) and daily without interruption, even when the cytotoxic chemotherapy is given on an intermittent schedule.
A dramatic single case report was published in 2015 of an advanced unresectable PDAC in a 64 y/o man, stage III, T4, N1, M0. His CA19-9 level was 189 units/ml (0–37 units/ml). He was given capecitabine, and two cycles of cisplatin. Then, after restaging he was still unresectable. Palliative gemcitabine and erlotinib was started but stopped after a month when pulmonary histoplasmosis was diagnosed. After a six month course of itraconazole 200 mg/day a repeat PET/CT showed a much reduced tumor size that was deemed resectable, A Whipple procedure was done. Five years later repeat evaluations showed no evidence of tumor [119]. Single case reports are not proof of anything but neither should they be ignored.
Azithromycin
The drug
Azithromycin is a broad-spectrum antibiotic that also has activity against several protists. Mechanism of action is by binding to microbial ribosomal RNA, stopping protein synthesis. It has a serum half-life of several days. Intracellular azithromycin levels are longer and are many times greater than serum levels. Neutrophil intracellular levels are several hundred times greater than serum levels [120]. Inhibition of autophagosome activity, lysosome leakage, and limiting dysfunctional inflammation are other azithromycin attributes seemingly independent of its antimicrobial activity [121].
Intersection with PDAC
Recent papers collected past research on the potential of azithromycin to interfere with aspects of malignant cell growth [121, 122].
Azithromycin inhibited lysosomal movement along microtubules by binding to tubulin but shows minimal cytotoxicity in vitro to non-transformed cells [123]. Azithromycin shows no evidence of cytotoxicity when used as antibiotic.
Empirical
Heterotopic non-small cell lung cancer tumors grew slower in azithromycin treated mice [123]. In vitro exposure to azithromycin gave significant cell death in cell lines of breast, ovarian, lung, pancreatic, and prostate cancers, and in glioblastoma and melanoma cell lines [124]. In another study of several lung cancer cell lines, azithromycin addition increased growth suppression of tyrosine kinases inhibitors and enhanced DNA damaging drugs’ carboplatin, doxorubicin and etoposide, cytotoxic effects. Importantly, non-transformed cells in culture were unaffected by azithromycin [125]. In this study azithromycin’s effect was secondary to creation of lysosomal damage and leakage. Specifically in PDAC cell lines azithromycin showed minimal cell number reduction after 48 hours co-incubation but with gefitinib but cell number reduction was 50% after coincubation with azithromycin at between 5 and 15 microM [126]. Azithromycin augmented cationic amphiphile lansoprazole’s cytotoxicity to squamous cell carcinoma and lung cancer cell lines. Evidence pointed to defective autophagosome function as the mechanism of action [127].
Autophagy
Autophagy is the catabolic process that takes place when an autophagosome fuses with a lysosome. Contained within lysosomes are many different proteases, nucleases, glycosidases, lipases, phospholipases, phosphatases, peptidases and sulfatases. A lysosomal membrane proton pump transfers protons from cytosol to lysosome, keeping lysosomes’ pH ~5, the optimum pH for the hydrolases. Lysosomes function to conserve amino acids, allowing their recycling. Accordingly, growth of squamous cell carcinoma cell lines in an amino acid sufficient culture medium becomes poor in the presence of azithromycin but goes back to baseline in an azithromycin containing medium with increased amino acid content [128].
Across the common cancers lysosomes tend to be more permeable than that of non-malignant cells. Since the common DNA damaging cancer chemotherapy drugs are sequestered in lysosomes, that relatively greater permeability is one of the origins of these drugs’ relatively selective cytotoxicity to malignant cells.
Anything that damages PDAC’s lysosomal membrane integrity will enhance cytotoxicity to a wide variety of pharmaceutical agents. Preclinical study of lysosomal permeabilization by experimental drugs sensitized PDAC’s cytotoxicity to gemcitabine [129, 130], to the HER1 (EGFR)/HER2 tyrosine kinase inhibitor lapatinib [131] to Ca++ release from damaged lysosomes [132], to tumor necrosis factor related apoptosis-inducing ligand (TRAIL) [133], and ro 5-fluorouracil [134, 135].
As discussed in section 4. above, PDAC is usually found to be nonsterile [111–116]. Many different microbes have been identified growing in PDAC tissue. If this colonization is causative or contributory to PDAC growth, metastasis, or chemotherapy resistance, this would be another advantage for adding azithromycin to standard gemcitabine.
Among cancers, PDAC is relatively resistant to autophagy and to apoptosis. The relationship between apoptosis and autophagy is not simple. Cell death occurs with excess autophagy as well as with inadequate autophagy function [136, 137].
Dapsone
The drug
Dapsone is one of the first of modern antibiotics, introduced in the 1930s to clinical practice. Used today as an antibiotic for treating Hansen’s disease, malaria, toxoplasmosis, and tuberculosis i.e., dapsone also has an interesting side effect of reducing neutrophil degranulation and chemotaxis. On that basis dapsone reduces neutrophil mediated tissue damage in diseases like the bullous pemphigus and the neutrophilic dermatoses [138–141]. Dapsone reduced IL-8 synthesis in several experimental settings [142, 143]. As a consequence of IL-8 reduction, dapsone can lower the neutrophil to lymphocyte ratio (NLR).
It is on these same bases of (a) limiting IL-8, (b) inhibiting neutrophil degranulation and (c) preventing neutrophil chemotaxis, that dapsone is used to treat the neutrophil mediated rash of EGFR inhibitors osimertinib and erlotinib [5, 144–146]. By virtue of these three attributes, dapsone is used to reduce neutrophil mediated lung damage in acute respiratory distress (ARDS) including the ARDS of COVID19 [147–149].
Neutrophils and NLR in PDAC
Neutrophils contain significant amounts of VEGF and other angiogenic factors, and they deliver these growth factors to tumors, including to PDAC [150–154].
Dozens of previous studies in PDAC have shown that survival becomes shorter as the neutrophil-to-lymphocyte ratio becomes higher. These studies were reviewed in 2021 [155]. Four new, independent studies reporting in 2023 have confirmed these past findings [156–159]. All these studies reflect the hundreds of studies showing this same association across all the common deadly cancers [160–163].
The tumor trophic function of neutrophils in PDAC [164–166] is reflected by a similar role in other common cancers [167–170]. PDAC both secrete into bloodstream and excrete into pancreatic juice significant amounts of IL-8 [171–179]. This IL-8 is trophic to both the malignant cells and their supportive stroma cells in PDAC.
The above data documents the established ability of dapsone to impede neutrophil accumulation in the neutrophilic dermatoses. By the same dapsone attributes, when carried over to PDAC, dapsone is projected to reduce the angiogenic, tumor trophic, and immunosuppressive functions of the IL-8 attracted tumor infiltrating and systemic neutrophils.
DISCUSSION AND CONCLUSION
Use of any untested experimental treatment involves risks. In the case of IPIAD, although the individual drugs are known to be of fairly low risk in their non-oncology use, the combination of these five drugs has never been tested. Although no particular drug-drug interaction is foreseen based on our extensive data on their pharmacology, surprises can’t be excluded.
Because of the many unknowns in the early clinical use of such untested regimens like IPIAD, careful follow up and monitoring are required to detect any untoward events. This means easy access 24 hours a day, 7 days a week by telephone to the prescribing physician with weekly in person meetings with review of systems, laboratory blood work, and addressing tolerability are minimum requirements.
The target doses listed in Table 3 are at the high end of tolerability. This is required by the hardiness of PDAC. Metastatic PDAC will not be slowed by half-measures. Using these target doses also becomes safer when the monitoring recommendations are followed. Given these considerations altogether and the alternatives available in 2023, continuous daily IPIAD added to standard gemcitabine, nab-paclitaxel, nab-paclitaxel must be regarded as a conservative approach to metastatic PDAC.
The reliability of relentless disease progression and short survival duration in metastatic PDAC plus our current lack of effective treatment to significantly lengthen survival, combined with the projected benign nature of continuous daily use of robust doses of the IPIAD drugs, altogether, warrant a pilot study of this regimen to run concomitantly with current standard cytotoxic treatments.
Abbreviations
ACEi: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; DHFR: dihydrofolate reductase; Hh: Hedgehog signaling; PDAC: pancreatic ductal adenocarcinoma; MDSC: myeloid derived suppressor cells; NLR: neutrophil to lymphocyte ratio.
CONFLICTS OF INTEREST
Author has no conflicts of interest to declare.
FUNDING
No funding was used for this paper.

- 1. Pathology of pancreatic cancer. Transl Gastroenterol Hepatol. 2019; 4:50. https://doi.org/10.21037/tgh.2019.06.02. PMID:31304427
- 2. Treatment of pancreatic cancer-neoadjuvant treatment in borderline resectable/locally advanced pancreatic cancer. Transl Gastroenterol Hepatol. 2019; 4:32. https://doi.org/10.21037/tgh.2019.04.09. PMID:31231699
- 3. Biological characteristics of pancreatic ductal adenocarcinoma: Initiation to malignancy, intracellular to extracellular. Cancer Lett. 2023; 574:216391. https://doi.org/10.1016/j.canlet.2023.216391. PMID:37714257
- 4. A phase Ib/IIa trial of 9 repurposed drugs combined with temozolomide for the treatment of recurrent glioblastoma: CUSP9v3. Neurooncol Adv. 2021; 3:vdab075. https://doi.org/10.1093/noajnl/vdab075. PMID:34377985
- 5. OPALS: A New Osimertinib Adjunctive Treatment of Lung Adenocarcinoma or Glioblastoma Using Five Repurposed Drugs. Cells. 2021; 10:1148. https://doi.org/10.3390/cells10051148. PMID:34068720
- 6. Blocking epithelial-to-mesenchymal transition in glioblastoma with a sextet of repurposed drugs: the EIS regimen. Oncotarget. 2017; 8:60727–49. https://doi.org/10.18632/oncotarget.18337. PMID:28977822
- 7. Toward a noncytotoxic glioblastoma therapy: blocking MCP-1 with the MTZ Regimen. Onco Targets Ther. 2016; 9:2535–45. https://doi.org/10.2147/OTT.S100407. PMID:27175087
- 8. Paths for Improving Bevacizumab Available in 2018: The ADZT Regimen for Better Glioblastoma Treatment. Med Sci (Basel). 2018; 6:84. https://doi.org/10.3390/medsci6040084. PMID:30274295
- 9. MDACT: A New Principle of Adjunctive Cancer Treatment Using Combinations of Multiple Repurposed Drugs, with an Example Regimen. Cancers (Basel). 2022; 14:2563. https://doi.org/10.3390/cancers14102563. PMID:35626167
- 10. Advances in pre-treatment evaluation of pancreatic ductal adenocarcinoma: a narrative review. J Gastrointest Oncol. 2023; 14:1114–30. https://doi.org/10.21037/jgo-22-1034. PMID:37201095
- 11. Current Status of the Diagnosis of Early-Stage Pancreatic Ductal Adenocarcinoma. Diagnostics (Basel). 2023; 13:215. https://doi.org/10.3390/diagnostics13020215. PMID:36673023
- 12. Defining the Optimal Duration of Neoadjuvant Therapy for Pancreatic Ductal Adenocarcinoma: Time for a Personalized Approach? Pancreas. 2022; 51:1083–91. https://doi.org/10.1097/MPA.0000000000002147. PMID:37078929
- 13. Role for Neoadjuvant Systemic Therapy for Potentially Resectable Pancreatic Cancer. Cancers (Basel). 2023; 15:2377. https://doi.org/10.3390/cancers15082377. PMID:37190305
- 14. Comparison of FOLFIRINOX vs Gemcitabine Plus Nab-Paclitaxel as First-Line Chemotherapy for Metastatic Pancreatic Ductal Adenocarcinoma. JAMA Netw Open. 2022; 5:e2216199. https://doi.org/10.1001/jamanetworkopen.2022.16199. PMID:35675073
- 15. Irbesartan (a comprehensive profile). Profiles Drug Subst Excip Relat Methodol. 2021; 46:185–272. https://doi.org/10.1016/bs.podrm.2020.07.004. PMID:33461698
- 16. Irbesartan: a review of its use in hypertension and in the management of diabetic nephropathy. Drugs. 2004; 64:999–1028. https://doi.org/10.2165/00003495-200464090-00011. PMID:15101793
- 17. Irbesartan overcomes gemcitabine resistance in pancreatic cancer by suppressing stemness and iron metabolism via inhibition of the Hippo/YAP1/c-Jun axis. J Exp Clin Cancer Res. 2023; 42:111. https://doi.org/10.1186/s13046-023-02671-8. PMID:37143164
- 18. The inhibition of renin-angiotensin system in advanced pancreatic cancer: an exploratory analysis in 349 patients. J Cancer Res Clin Oncol. 2015; 141:933–39. https://doi.org/10.1007/s00432-014-1873-2. PMID:25398651
- 19. Inhibition of renin-angiotensin system affects prognosis of advanced pancreatic cancer receiving gemcitabine. Br J Cancer. 2010; 103:1644–48. https://doi.org/10.1038/sj.bjc.6605955. PMID:20978506
- 20. The use of angiotensin system inhibitors correlates with longer survival in resected pancreatic adenocarcinoma patients. HPB (Oxford). 2023; 25:320–29. https://doi.org/10.1016/j.hpb.2022.12.002. PMID:36610939
- 21. Angiotensin blockade therapy and survival in pancreatic cancer: a population study. BMC Cancer. 2022; 22:150. https://doi.org/10.1186/s12885-022-09200-4. PMID:35130875
- 22. Impact of Angiotensin Receptor Blocker Use on Overall Survival Among Patients Undergoing Resection for Pancreatic Cancer. World J Surg. 2017; 41:2361–70. https://doi.org/10.1007/s00268-017-4021-8. PMID:28429090
- 23. A multicenter phase II trial of gemcitabine and candesartan combination therapy in patients with advanced pancreatic cancer: GECA2. Invest New Drugs. 2013; 31:1294–99. https://doi.org/10.1007/s10637-013-9972-5. PMID:23690239
- 24. Angiotensin converting enzyme-independent, local angiotensin II-generation in human pancreatic ductal cancer tissues. Int J Oncol. 2003; 23:593–98. PMID:12888892
- 25. Synergistic inhibitory effect of gemcitabine and angiotensin type-1 receptor blocker, losartan, on murine pancreatic tumor growth via anti-angiogenic activities. Oncol Rep. 2009; 22:355–60. PMID:19578777
- 26. Antitumor effect of angiotensin II type 1 receptor blocker losartan for orthotopic rat pancreatic adenocarcinoma. Pancreas. 2014; 43:886–90. https://doi.org/10.1097/MPA.0000000000000125. PMID:24717824
- 27. MicroRNA profiles following telmisartan treatment in pancreatic ductal adenocarcinoma cells. J Cancer Res Ther. 2022; 18:S305–12. https://doi.org/10.4103/jcrt.JCRT_104_20. PMID:36510981
- 28. Angiotensin-converting Enzyme Inhibitors and Angiotensin Receptor Blockers as Potential Therapeutic Options for Pancreatic Cancer. Curr Cancer Drug Targets. 2022; 22:785–95. https://doi.org/10.2174/1568009622666220517104411. PMID:35585824
- 29. Association of losartan with outcomes in metastatic pancreatic cancer patients treated with chemotherapy. J Clin Transl Res. 2021; 7:257–62. PMID:34104829
- 30. The physiology of a local renin-angiotensin system in the pancreas. J Physiol. 2007; 580:31–37. https://doi.org/10.1113/jphysiol.2006.126193. PMID:17218353
- 31. Therapeutic Targeting of Cancer Stem Cells via Modulation of the Renin-Angiotensin System. Front Oncol. 2019; 9:745. https://doi.org/10.3389/fonc.2019.00745. PMID:31440473
- 32. Impact of sarcopenia on prediction of progression-free survival and overall survival of patients with pancreatic ductal adenocarcinoma receiving first-line gemcitabine and nab-paclitaxel chemotherapy. Pancreatology. 2022; 22:277–85. https://doi.org/10.1016/j.pan.2021.12.013. PMID:35033425
- 33. Sarcopenia: Prognostic Value for Unresectable Pancreatic Ductal Adenocarcinoma Patients Treated With Gemcitabine Plus Nab-Paclitaxel. Pancreas. 2022; 51:148–52. https://doi.org/10.1097/MPA.0000000000001985. PMID:35404889
- 34. Angiotensin II inhibition: a potential treatment to slow the progression of sarcopenia. Clin Sci (Lond). 2021; 135:2503–20. https://doi.org/10.1042/CS20210719. PMID:34751393
- 35. Mini-review: Angiotensin- converting enzyme 1 (ACE1) and the impact for diseases such as Alzheimer’s disease, sarcopenia, cancer, and COVID-19. Front Aging. 2023; 4:1117502. https://doi.org/10.3389/fragi.2023.1117502. PMID:36756193
- 36. Angiotensin II receptor blocker and statin combination therapy associated with higher skeletal muscle index in patients with cardiovascular disease: A retrospective study. J Clin Pharm Ther. 2022; 47:89–96. https://doi.org/10.1111/jcpt.13540. PMID:34668212
- 37. Angiotensin II receptor blockade is associated with preserved muscle strength in chronic hemodialysis patients. BMC Nephrol. 2019; 20:54. https://doi.org/10.1186/s12882-019-1223-3. PMID:30764799
- 38. Effects of losartan and exercise on muscle mass and exercise endurance of old mice. Exp Gerontol. 2022; 165:111869. https://doi.org/10.1016/j.exger.2022.111869. PMID:35710057
- 39. Screening of DHFR-binding drugs by MALDI-TOFMS. Anal Bioanal Chem. 2008; 392:1335–44. https://doi.org/10.1007/s00216-008-2409-x. PMID:18841351
- 40. Characterization of a lipophilic antifolate resistance provoked by treatment of mammalian cells with the antiparasitic agent pyrimethamine. J Biol Chem. 1993; 268:4556–66. PMID:8440739
- 41. Dysregulation of de novo nucleotide biosynthetic pathway enzymes in cancer and targeting opportunities. Cancer Lett. 2020; 470:134–40. https://doi.org/10.1016/j.canlet.2019.11.013. PMID:31733288
- 42. A Systematic Review of High-Dose Methotrexate for Adults with Primary Central Nervous System Lymphoma. Cancers (Basel). 2023; 15:1459. https://doi.org/10.3390/cancers15051459. PMID:36900250
- 43. A comprehensive review on methotrexate containing nanoparticles; an appropriate tool for cancer treatment. Mol Biol Rep. 2022; 49:11049–60. https://doi.org/10.1007/s11033-022-07782-7. PMID:36097117
- 44. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006; 5:949–60. https://doi.org/10.1016/S1474-4422(06)70598-1. PMID:17052662
- 45. Adverse Event Profile of Pyrimethamine-Based Therapy in Toxoplasmosis: A Systematic Review. Drugs R D. 2017; 17:523–44. https://doi.org/10.1007/s40268-017-0206-8. PMID:28879584
- 46. Pyrimethamine as a Potent and Selective Inhibitor of Acute Myeloid Leukemia Identified by High-throughput Drug Screening. Curr Cancer Drug Targets. 2016; 16:818–28. https://doi.org/10.2174/1568009616666160617103301. PMID:27321378
- 47. Pyrimethamine in the myeloproliferative disorders: a forgotten treatment? Clin Lab Haematol. 1987; 9:129–36. https://doi.org/10.1111/j.1365-2257.1987.tb01394.x. PMID:3621857
- 48. Targeting constitutively active STAT3 in chronic lymphocytic leukemia: A clinical trial of the STAT3 inhibitor pyrimethamine with pharmacodynamic analyses. Am J Hematol. 2021; 96:E95–98. https://doi.org/10.1002/ajh.26084. PMID:33373063
- 49. Pyrimethamine in prevention of relapses of meningeal leukemia: report of two cases. Cancer. 1978; 42:1216–18. https://doi.org/10.1002/1097-0142(197809)42:3<1216::aid-cncr2820420327>3.0.co;2-f. PMID:279392
- 50. Importance of myeloid derived suppressor cells in cancer from a biomarker perspective. Cell Immunol. 2021; 361:104280. https://doi.org/10.1016/j.cellimm.2020.104280. PMID:33445053
- 51. Myeloid-derived suppressor cells: an emerging target for anticancer immunotherapy. Mol Cancer. 2022; 21:184. https://doi.org/10.1186/s12943-022-01657-y. PMID:36163047
- 52. Myeloid-Derived Suppressor Cells in Solid Tumors. Cells. 2022; 11:310. https://doi.org/10.3390/cells11020310. PMID:35053426
- 53. Myeloid Cell-Derived Arginase in Cancer Immune Response. Front Immunol. 2020; 11:938. https://doi.org/10.3389/fimmu.2020.00938. PMID:32499785
- 54. STAT3 signaling in pancreatic ductal adenocarcinoma: a candidate therapeutic target. Pathol Res Pract. 2023; 245:154425. https://doi.org/10.1016/j.prp.2023.154425. PMID:37019018
- 55. IGFBP2 Drives Regulatory T Cell Differentiation through STAT3/IDO Signaling Pathway in Pancreatic Cancer. J Pers Med. 2022; 12:2005. https://doi.org/10.3390/jpm12122005. PMID:36556226
- 56. Pancreatic cancer-derived small extracellular vesical ezrin activates fibroblasts to exacerbate cancer metastasis through STAT3 and YAP-1 signaling pathways. Mol Oncol. 2023; 17:1628–47. https://doi.org/10.1002/1878-0261.13442. PMID:37171030
- 57. Advances in the role of STAT3 in macrophage polarization. Front Immunol. 2023; 14:1160719. https://doi.org/10.3389/fimmu.2023.1160719. PMID:37081874
- 58. Regulation and therapy, the role of JAK2/STAT3 signaling pathway in OA: a systematic review. Cell Commun Signal. 2023; 21:67. https://doi.org/10.1186/s12964-023-01094-4. PMID:37013568
- 59. The Physiological and Pathophysiological Role of IL-6/STAT3-Mediated Signal Transduction and STAT3 Binding Partners in Therapeutic Applications. Biol Pharm Bull. 2023; 46:364–78. https://doi.org/10.1248/bpb.b22-00887. PMID:36858565
- 60. Myeloid derived suppressor cells-An overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology. 2015; 4:e954829. https://doi.org/10.4161/21624011.2014.954829. PMID:25949858
- 61. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013; 73:3007–18. https://doi.org/10.1158/0008-5472.CAN-12-4601. PMID:23514705
- 62. Response to radiotherapy in pancreatic ductal adenocarcinoma is enhanced by inhibition of myeloid-derived suppressor cells using STAT3 anti-sense oligonucleotide. Cancer Immunol Immunother. 2021; 70:989–1000. https://doi.org/10.1007/s00262-020-02701-w. PMID:33097963
- 63. Immunosuppression by monocytic myeloid-derived suppressor cells in patients with pancreatic ductal carcinoma is orchestrated by STAT3. J Immunother Cancer. 2019; 7:255. https://doi.org/10.1186/s40425-019-0734-6. PMID:31533831
- 64. Metabolic reprogramming of immune cells in pancreatic cancer progression. Biomed Pharmacother. 2023; 157:113992. https://doi.org/10.1016/j.biopha.2022.113992. PMID:36395610
- 65. Inhibition of myeloid-derived suppressor cell arginase-1 production enhances T-cell-based immunotherapy against Cryptococcus neoformans infection. Nat Commun. 2022; 13:4074. https://doi.org/10.1038/s41467-022-31723-4. PMID:35835754
- 66. Immunosuppressive effects and mechanisms of three myeloid-derived suppressor cells subsets including monocytic-myeloid-derived suppressor cells, granulocytic-myeloid-derived suppressor cells, and immature-myeloid-derived suppressor cells. J Cancer Res Ther. 2021; 17:1093–100. https://doi.org/10.4103/jcrt.JCRT_1222_20. PMID:34528569
- 67. Neutralization of NET-associated human ARG1 enhances cancer immunotherapy. Sci Transl Med. 2023; 15:eabq6221. https://doi.org/10.1126/scitranslmed.abq6221. PMID:36921034
- 68. Arginase 1 is a key driver of immune suppression in pancreatic cancer. Elife. 2023; 12:e80721. https://doi.org/10.7554/eLife.80721. PMID:36727849
- 69. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008; 205:2235–49. https://doi.org/10.1084/jem.20080132. PMID:18809714
- 70. Targeting STAT3 in adoptively transferred T cells promotes their in vivo expansion and antitumor effects. Cancer Res. 2010; 70:9599–610. https://doi.org/10.1158/0008-5472.CAN-10-1293. PMID:21118964
- 71. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005; 11:1314–21. https://doi.org/10.1038/nm1325. PMID:16288283
- 72. Targeting Stat3 in the myeloid compartment drastically improves the in vivo antitumor functions of adoptively transferred T cells. Cancer Res. 2010; 70:7455–64. https://doi.org/10.1158/0008-5472.CAN-10-0736. PMID:20841481
- 73. The STAT3 inhibitor pyrimethamine displays anti-cancer and immune stimulatory effects in murine models of breast cancer. Cancer Immunol Immunother. 2018; 67:13–23. https://doi.org/10.1007/s00262-017-2057-0. PMID:28875329
- 74. Inhibitors of the Transcription Factor STAT3 Decrease Growth and Induce Immune Response Genes in Models of Malignant Pleural Mesothelioma (MPM). Cancers (Basel). 2020; 13:7. https://doi.org/10.3390/cancers13010007. PMID:33374980
- 75. Targeted elimination of myeloid-derived suppressor cells via regulation of the STAT pathway alleviates tumor immunosuppression in neuroblastoma. Immunol Lett. 2021; 240:31–40. https://doi.org/10.1016/j.imlet.2021.09.011. PMID:34600949
- 76. Tumor NLRP3-Derived IL-1β Drives the IL-6/STAT3 Axis Resulting in Sustained MDSC-Mediated Immunosuppression. Front Immunol. 2021; 12:661323. https://doi.org/10.3389/fimmu.2021.661323. PMID:34531850
- 77. The role of STAT3 in tumor-mediated immune suppression. J Neurooncol. 2015; 123:385–94. https://doi.org/10.1007/s11060-015-1731-3. PMID:25700834
- 78. STAT3 inhibition enhances CDN-induced STING signaling and antitumor immunity. Cancer Lett. 2019; 450:110–22. https://doi.org/10.1016/j.canlet.2019.02.029. PMID:30790684
- 79. STAT3 Silencing and TLR7/8 Pathway Activation Repolarize and Suppress Myeloid-Derived Suppressor Cells From Breast Cancer Patients. Front Immunol. 2020; 11:613215. https://doi.org/10.3389/fimmu.2020.613215. PMID:33679700
- 80. STAT3 inhibitor Napabucasin abrogates MDSC immunosuppressive capacity and prolongs survival of melanoma-bearing mice. J Immunother Cancer. 2022; 10:e004384. https://doi.org/10.1136/jitc-2021-004384. PMID:35301236
- 81. Antimalarial Drug Pyrimethamine Plays a Dual Role in Antitumor Proliferation and Metastasis through Targeting DHFR and TP. Mol Cancer Ther. 2019; 18:541–55. https://doi.org/10.1158/1535-7163.MCT-18-0936. PMID:30642883
- 82. Multifunctional role of thymidine phosphorylase in cancer. Trends Cancer. 2022; 8:482–93. https://doi.org/10.1016/j.trecan.2022.01.018. PMID:35193822
- 83. The multifaceted antineoplastic role of pyrimethamine against human malignancies. IUBMB Life. 2022; 74:198–212. https://doi.org/10.1002/iub.2590. PMID:34921584
- 84. Thymidine phosphorylase in cancer aggressiveness and chemoresistance. Pharmacol Res. 2018; 132:15–20. https://doi.org/10.1016/j.phrs.2018.03.019. PMID:29604437
- 85. Expression of thymidine phosphorylase is associated with a poor prognosis in patients with ductal adenocarcinoma of the pancreas. Clin Cancer Res. 1998; 4:1619–24. PMID:9676835
- 86. Heterogeneic distribution of thymidine phosphorylase between primary tumors and metastatic lesions of human pancreatic ductal carcinoma: implications for the efficacy of chemotherapy with 5-FU or its derivatives. Cancer Chemother Pharmacol. 2001; 47:415–22. https://doi.org/10.1007/s002800000252. PMID:11391857
- 87. Angiogenesis in pancreatic carcinoma: thymidine phosphorylase expression in stromal cells and intratumoral microvessel density as independent predictors of overall and relapse-free survival. Cancer. 2001; 92:1788–97. https://doi.org/10.1002/1097-0142(20011001)92:7<1788::aid-cncr1695>3.0.co;2-z. PMID:11745251
- 88. Prognostic role of angiogenesis and its correlations with thymidine phosphorylase and p53 expression in ductal adenocarcinoma of the pancreas. Hepatogastroenterology. 2007; 54:1635–40. PMID:18019682
- 89. Prognostic value of thymidine phosphorylase expression for pancreatic cancer. Hepatogastroenterology. 2009; 56:1178–82. PMID:19760965
- 90. Repurposed itraconazole for use in the treatment of malignancies as a promising therapeutic strategy. Biomed Pharmacother. 2022; 154:113616. https://doi.org/10.1016/j.biopha.2022.113616. PMID:36055112
- 91. Mode of action of itraconazole: morphological aspects. Mycoses. 1989 (Suppl 1); 32:53–59. https://doi.org/10.1111/j.1439-0507.1989.tb02294.x. PMID:2561185
- 92. Repurposing Drugs in Oncology (ReDO)-itraconazole as an anti-cancer agent. Ecancermedicalscience. 2015; 9:521. https://doi.org/10.3332/ecancer.2015.521. PMID:25932045
- 93. Combination Chemotherapy with Itraconazole for Treating Metastatic Pancreatic Cancer in the Second-line or Additional Setting. Anticancer Res. 2015; 35:4191–96. PMID:26124377
- 94. Itraconazole inhibits proliferation of pancreatic cancer cells through activation of Bak-1. J Cell Biochem. 2019; 120:4333–41. https://doi.org/10.1002/jcb.27719. PMID:30260036
- 95. Itraconazole inhibits invasion and migration of pancreatic cancer cells by suppressing TGF-β/SMAD2/3 signaling. Oncol Rep. 2018; 39:1573–82. https://doi.org/10.3892/or.2018.6281. PMID:29484419
- 96. Inhibition of the hedgehog pathway for the treatment of cancer using Itraconazole. Onco Targets Ther. 2019; 12:6875–86. https://doi.org/10.2147/OTT.S223119. PMID:31692536
- 97. Formulation and evaluation of itraconazole liposomes for Hedgehog pathway inhibition. J Liposome Res. 2020; 30:305–11. https://doi.org/10.1080/08982104.2019.1668011. PMID:31576768
- 98. Itraconazole inhibits the Hedgehog signaling pathway thereby inducing autophagy-mediated apoptosis of colon cancer cells. Cell Death Dis. 2020; 11:539. https://doi.org/10.1038/s41419-020-02742-0. PMID:32681018
- 99. “Hedgehog pathway”: a potential target of itraconazole in the treatment of cancer. J Cancer Res Clin Oncol. 2020; 146:297–304. https://doi.org/10.1007/s00432-019-03117-5. PMID:31960187
- 100. Cellular and molecular mechanisms of Hedgehog signalling. Nat Rev Mol Cell Biol. 2023; 24:668–87. https://doi.org/10.1038/s41580-023-00591-1. PMID:36932157
- 101. Regulation of hematopoiesis by hedgehog signaling (Review). Mol Med Rep. 2023; 27:100. https://doi.org/10.3892/mmr.2023.12987. PMID:36999588
- 102. The emerging roles of Hedgehog signaling in tumor immune microenvironment. Front Oncol. 2023; 13:1171418. https://doi.org/10.3389/fonc.2023.1171418. PMID:37213270
- 103. The pancreas cancer microenvironment. Clin Cancer Res. 2012; 18:4266–76. https://doi.org/10.1158/1078-0432.CCR-11-3114. PMID:22896693
- 104. Sonic Hedgehog Signaling in Organogenesis, Tumors, and Tumor Microenvironments. Int J Mol Sci. 2020; 21:758. https://doi.org/10.3390/ijms21030758. PMID:31979397
- 105. Hedgehog signaling promotes angiogenesis directly and indirectly in pancreatic cancer. Angiogenesis. 2020; 23:479–92. https://doi.org/10.1007/s10456-020-09725-x. PMID:32444947
- 106. Hedgehog signaling: from the cuirass to the heart of pancreatic cancer. Pancreatology. 2012; 12:388–93. https://doi.org/10.1016/j.pan.2012.06.001. PMID:22898642
- 107. Hedgehog Signaling in Pancreatic Fibrosis and Cancer. Medicine (Baltimore). 2016; 95:e2996. https://doi.org/10.1097/MD.0000000000002996. PMID:26962810
- 108. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn J Basic Med Sci. 2018; 18:8–20. https://doi.org/10.17305/bjbms.2018.2756. PMID:29274272
- 109. Hedgehog Signaling and Truncated GLI1 in Cancer. Cells. 2020; 9:2114. https://doi.org/10.3390/cells9092114. PMID:32957513
- 110. Hedgehog Pathway Inhibitors against Tumor Microenvironment. Cells. 2021; 10:3135. https://doi.org/10.3390/cells10113135. PMID:34831357
- 111. Micro- and Mycobiota Dysbiosis in Pancreatic Ductal Adenocarcinoma Development. Cancers (Basel). 2021; 13:3431. https://doi.org/10.3390/cancers13143431. PMID:34298645
- 112. Implications of the microbiome in the development and treatment of pancreatic cancer: Thinking outside of the box by looking inside the gut. Neoplasia. 2021; 23:246–56. https://doi.org/10.1016/j.neo.2020.12.008. PMID:33418277
- 113. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019; 574:264–67. https://doi.org/10.1038/s41586-019-1608-2. PMID:31578522
- 114. The immune response to a fungus in pancreatic cancer samples. bioRxiv. 2023. [Epub ahead of print]. https://doi.org/10.1101/2023.03.28.534606. PMID:37034706
- 115. Complement and Fungal Dysbiosis as Prognostic Markers and Potential Targets in PDAC Treatment. Curr Oncol. 2022; 29:9833–54. https://doi.org/10.3390/curroncol29120773. PMID:36547187
- 116. Intratumoral Malasseziaglobosa Levels Predict Survival and Therapeutic Response to Adjuvant Chemotherapy in Patients With Pancreatic Ductal Adenocarcinoma. Gastroenterology. 2023; 165:502–4.e2. https://doi.org/10.1053/j.gastro.2023.04.017. PMID:37119937
- 117. First-Line Gemcitabine, Nab-Paclitaxel, and Oxaliplatin Chemotherapy With Itraconazole in Patients With Metastatic Pancreatic Cancer: A Single Institution Experience. Anticancer Res. 2022; 42:6063–69. https://doi.org/10.21873/anticanres.16118. PMID:36456160
- 118. Itraconazole inhibits endothelial cell migration by disrupting inositol pyrophosphate-dependent focal adhesion dynamics and cytoskeletal remodeling. Biomed Pharmacother. 2023; 161:114449. https://doi.org/10.1016/j.biopha.2023.114449. PMID:36857911
- 119. Itraconazole therapy in a pancreatic adenocarcinoma patient: A case report. J Oncol Pharm Pract. 2016; 22:528–32. https://doi.org/10.1177/1078155215572931. PMID:25670260
- 120. Nonantimicrobial Actions of Macrolides: Overview and Perspectives for Future Development. Pharmacol Rev. 2021; 73:233–62. https://doi.org/10.1124/pharmrev.121.000300. PMID:34716226
- 121. Immunomodulatory role of azithromycin: Potential applications to radiation-induced lung injury. Front Oncol. 2023; 13:966060. https://doi.org/10.3389/fonc.2023.966060. PMID:36969016
- 122. Targeting Cancer Stem Cells with Repurposed Drugs to Improve Current Therapies. Recent Pat Anticancer Drug Discov. 2021; 16:136–60. https://doi.org/10.2174/1574892816666210208232251. PMID:33563159
- 123. Azithromycin, a potent autophagy inhibitor for cancer therapy, perturbs cytoskeletal protein dynamics. Br J Cancer. 2023; 128:1838–49. https://doi.org/10.1038/s41416-023-02210-4. PMID:36871041
- 124. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: treating cancer like an infectious disease. Oncotarget. 2015; 6:4569–84. https://doi.org/10.18632/oncotarget.3174. PMID:25625193
- 125. Azithromycin enhances the cytotoxicity of DNA-damaging drugs via lysosomal membrane permeabilization in lung cancer cells. Cancer Sci. 2021; 112:3324–37. https://doi.org/10.1111/cas.14992. PMID:34051014
- 126. Macrolides sensitize EGFR-TKI-induced non-apoptotic cell death via blocking autophagy flux in pancreatic cancer cell lines. Int J Oncol. 2016; 48:45–54. https://doi.org/10.3892/ijo.2015.3237. PMID:26718641
- 127. Macrolide antibiotics enhance the antitumor effect of lansoprazole resulting in lysosomal membrane permeabilization-associated cell death. Int J Oncol. 2020; 57:1280–92. https://doi.org/10.3892/ijo.2020.5138. PMID:33173988
- 128. Macrolide Antibiotics Exhibit Cytotoxic Effect under Amino Acid-Depleted Culture Condition by Blocking Autophagy Flux in Head and Neck Squamous Cell Carcinoma Cell Lines. PLoS One. 2016; 11:e0164529. https://doi.org/10.1371/journal.pone.0164529. PMID:27977675
- 129. CQ sensitizes human pancreatic cancer cells to gemcitabine through the lysosomal apoptotic pathway via reactive oxygen species. Mol Oncol. 2018; 12:529–44. https://doi.org/10.1002/1878-0261.12179. PMID:29453806
- 130. NDRG1 suppresses basal and hypoxia-induced autophagy at both the initiation and degradation stages and sensitizes pancreatic cancer cells to lysosomal membrane permeabilization. Biochim Biophys Acta Gen Subj. 2020; 1864:129625. https://doi.org/10.1016/j.bbagen.2020.129625. PMID:32335136
- 131. Lysosome-targeted drug combination induces multiple organelle dysfunctions and non-canonical death in pancreatic cancer cells. Oncol Rep. 2022; 47:40. https://doi.org/10.3892/or.2021.8251. PMID:34958115
- 132. Inhibition of Sp1 prevents ER homeostasis and causes cell death by lysosomal membrane permeabilization in pancreatic cancer. Sci Rep. 2017; 7:1564. https://doi.org/10.1038/s41598-017-01696-2. PMID:28484232
- 133. Triptolide sensitizes pancreatic cancer cells to TRAIL-induced activation of the death receptor pathway. Cancer Lett. 2014; 348:156–66. https://doi.org/10.1016/j.canlet.2014.03.016. PMID:24662747
- 134. Oleanolic acid arrests cell cycle and induces apoptosis via ROS-mediated mitochondrial depolarization and lysosomal membrane permeabilization in human pancreatic cancer cells. J Appl Toxicol. 2013; 33:756–65. https://doi.org/10.1002/jat.2725. PMID:22678527
- 135. Oleanolic acid potentiates the antitumor activity of 5-fluorouracil in pancreatic cancer cells. Oncol Rep. 2012; 28:1339–45. https://doi.org/10.3892/or.2012.1921. PMID:22825128
- 136. Lysosomes as a Target of Anticancer Therapy. Int J Mol Sci. 2023; 24:2176. https://doi.org/10.3390/ijms24032176. PMID:36768500
- 137. Therapeutic strategies of drug repositioning targeting autophagy to induce cancer cell death: from pathophysiology to treatment. J Hematol Oncol. 2017; 10:67. https://doi.org/10.1186/s13045-017-0436-9. PMID:28279189
- 138. Update on the use of dapsone in dermatology. Int J Dermatol. 2020; 59:787–95. https://doi.org/10.1111/ijd.14761. PMID:31909480
- 139. A comprehensive insight into the anti-inflammatory properties of dapsone. Naunyn Schmiedebergs Arch Pharmacol. 2022; 395:1509–23. https://doi.org/10.1007/s00210-022-02297-1. PMID:36125533
- 140. Erlotinib augmentation with dapsone for rash mitigation and increased anti-cancer effectiveness. Springerplus. 2015; 4:638. https://doi.org/10.1186/s40064-015-1441-5. PMID:26543772
- 141. Dapsone, More than an Effective Neuro and Cytoprotective Drug. Curr Neuropharmacol. 2022; 20:194–210. https://doi.org/10.2174/1570159X19666210617143108. PMID:34139984
- 142. Early treatment with dapsone after spinal cord injury in rats decreases the inflammatory response and promotes long-term functional recovery. Heliyon. 2023; 9:e14687. https://doi.org/10.1016/j.heliyon.2023.e14687. PMID:37009237
- 143. Anti-glioma Activity of Dapsone and Its Enhancement by Synthetic Chemical Modification. Neurochem Res. 2017; 42:3382–89. https://doi.org/10.1007/s11064-017-2378-6. PMID:28852934
- 144. Osimertinib-Induced Cutaneous Vasculitis Responsive to Low-Dose Dapsone Without Interruption of Anticancer Therapy: A Case Report and Review of the Literature. JTO Clin Res Rep. 2022; 3:100415. https://doi.org/10.1016/j.jtocrr.2022.100415. PMID:36275908
- 145. Severe EGFR inhibitor-induced acneiform eruption responding to dapsone. Dermatol Online J. 2021; 27. https://doi.org/10.5070/D327754366. PMID:34391331
- 146. The strange connection between epidermal growth factor receptor tyrosine kinase inhibitors and dapsone: from rash mitigation to the increase in anti-tumor activity. Curr Med Res Opin. 2016; 32:1839–48. https://doi.org/10.1080/03007995.2016.1211522. PMID:27398628
- 147. Dapsone as treatment adjunct in ARDS. Exp Lung Res. 2020; 46:157–61. https://doi.org/10.1080/01902148.2020.1753266. PMID:32286085
- 148. Benefits of Using Dapsone in Patients Hospitalized with COVID-19. Vaccines (Basel). 2022; 10:195. https://doi.org/10.3390/vaccines10020195. PMID:35214654
- 149. Dapsone Lowers Neutrophil to Lymphocyte Ratio and Mortality in COVID-19 Patients Admitted to the ICU. Int J Mol Sci. 2022; 23:15563. https://doi.org/10.3390/ijms232415563. PMID:36555204
- 150. Contribution to Tumor Angiogenesis From Innate Immune Cells Within the Tumor Microenvironment: Implications for Immunotherapy. Front Immunol. 2018; 9:527. https://doi.org/10.3389/fimmu.2018.00527. PMID:29675018
- 151. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017; 17:457–74. https://doi.org/10.1038/nrc.2017.51. PMID:28706266
- 152. Myeloid Derived Suppressor Cells Interactions With Natural Killer Cells and Pro-angiogenic Activities: Roles in Tumor Progression. Front Immunol. 2019; 10:771. https://doi.org/10.3389/fimmu.2019.00771. PMID:31057536
- 153. Why dapsone stops seizures and may stop neutrophils’ delivery of VEGF to glioblastoma. Br J Neurosurg. 2012; 26:813–17. https://doi.org/10.3109/02688697.2012.674577. PMID:22551309
- 154. The recent progress of myeloid-derived suppressor cell and its targeted therapies in cancers. MedComm (2020). 2023; 4:e323. https://doi.org/10.1002/mco2.323. PMID:37547175
- 155. Peripheral Blood Cell Variables Related to Systemic Inflammation in Patients With Unresectable or Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis. Pancreas. 2021; 50:1131–36. https://doi.org/10.1097/MPA.0000000000001878. PMID:34714276
- 156. DUPAN-2 as a Risk Factor of Early Recurrence After Curative Pancreatectomy for Patients With Pancreatic Ductal Adenocarcinoma. Pancreas. 2023; 52:e110–14. https://doi.org/10.1097/MPA.0000000000002209. PMID:37523601
- 157. Real-Life Results of Palliative Chemotherapy in Metastatic Pancreatic Ductal Adenocarcinoma. Cancers (Basel). 2023; 15:3500. https://doi.org/10.3390/cancers15133500. PMID:37444612
- 158. Utility of Established Prognostic Scoring Systems for Patients with Advanced Pancreatic Adenocarcinoma Enrolled in Immunotherapy-Based Early-Phase Clinical Trials. J Gastrointest Cancer. 2023; 54:1308–15. https://doi.org/10.1007/s12029-023-00930-7. PMID:37119430
- 159. Systemic inflammatory prognostic scores in advanced pancreatic adenocarcinoma. Br J Cancer. 2023; 128:1916–21. https://doi.org/10.1038/s41416-023-02214-0. PMID:36927977
- 160. Prognostic Values of Systemic Inflammatory Immunological Markers in Glioblastoma: A Systematic Review and Meta-Analysis. Cancers (Basel). 2023; 15:3339. https://doi.org/10.3390/cancers15133339. PMID:37444448
- 161. Focus on the Dynamics of Neutrophil-to-Lymphocyte Ratio in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Meta-Analysis and Systematic Review. Cancers (Basel). 2022; 14:5297. https://doi.org/10.3390/cancers14215297. PMID:36358716
- 162. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. 2021; 14:173. https://doi.org/10.1186/s13045-021-01187-y. PMID:34674757
- 163. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014; 106:dju124. https://doi.org/10.1093/jnci/dju124. PMID:24875653
- 164. Tumor Microenvironment Remodeling Enables Bypass of Oncogenic KRAS Dependency in Pancreatic Cancer. Cancer Discov. 2020; 10:1058–77. https://doi.org/10.1158/2159-8290.CD-19-0597. PMID:32341020
- 165. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015; 34:2219–36. https://doi.org/10.15252/embj.201490147. PMID:26136213
- 166. PDAC, the Influencer Cancer: Cross-Talk with Tumor Microenvironment and Connected Potential Therapy Strategies. Cancers (Basel). 2023; 15:2923. https://doi.org/10.3390/cancers15112923. PMID:37296886
- 167. Inflammatory Responses during Tumour Initiation: From Zebrafish Transgenic Models of Cancer to Evidence from Mouse and Man. Cells. 2020; 9:1018. https://doi.org/10.3390/cells9041018. PMID:32325966
- 168. Neutrophil: A New Player in Metastatic Cancers. Front Immunol. 2020; 11:565165. https://doi.org/10.3389/fimmu.2020.565165. PMID:33101283
- 169. Neutrophils: important contributors to tumor progression and metastasis. Cancer Metastasis Rev. 2015; 34:735–51. https://doi.org/10.1007/s10555-015-9594-9. PMID:26361774
- 170. Research Supporting a Pilot Study of Metronomic Dapsone during Glioblastoma Chemoirradiation. Med Sci (Basel). 2021; 9:12. https://doi.org/10.3390/medsci9010012. PMID:33669324
- 171. Protein biomarkers in pancreatic juice and serum for identification of pancreatic cancer. Gastrointest Endosc. 2022; 96:801–13.e2. https://doi.org/10.1016/j.gie.2022.04.1342. PMID:35537661
- 172. Serum biomarker panel diagnostics in pancreatic ductal adenocarcinoma: the clinical utility of soluble interleukins, IFN-γ, TNF-α and PD-1/PD-L1 in comparison to established serum tumor markers. J Cancer Res Clin Oncol. 2023; 149:2463–74. https://doi.org/10.1007/s00432-022-04112-z. PMID:35737090
- 173. Addition of Losartan to FOLFIRINOX and Chemoradiation Reduces Immunosuppression-Associated Genes, Tregs, and FOXP3+ Cancer Cells in Locally Advanced Pancreatic Cancer. Clin Cancer Res. 2023; 29:1605–19. https://doi.org/10.1158/1078-0432.CCR-22-1630. PMID:36749873
- 174. Fusobacterium nucleatum induces proliferation and migration in pancreatic cancer cells through host autocrine and paracrine signaling. Sci Signal. 2022; 15:eabn4948. https://doi.org/10.1126/scisignal.abn4948. PMID:36256708
- 175. Pancreatic Ductal Adenocarcinoma (PDAC) circulating tumor cells influence myeloid cell differentiation to support their survival and immunoresistance in portal vein circulation. PLoS One. 2022; 17:e0265725. https://doi.org/10.1371/journal.pone.0265725. PMID:35316296
- 176. Systemic inflammation is a determinant of outcomes of CD40 agonist-based therapy in pancreatic cancer patients. JCI Insight. 2021; 6:145389. https://doi.org/10.1172/jci.insight.145389. PMID:33497362
- 177. Metastasis-associated fibroblasts promote angiogenesis in metastasized pancreatic cancer via the CXCL8 and the CCL2 axes. Sci Rep. 2020; 10:5420. https://doi.org/10.1038/s41598-020-62416-x. PMID:32214219
- 178. Inflammatory cytokines and combined biomarker panels in pancreatic ductal adenocarcinoma: Enhancing diagnostic accuracy. PLoS One. 2019; 14:e0221169. https://doi.org/10.1371/journal.pone.0221169. PMID:31415645
- 179. BAG3-positive pancreatic stellate cells promote migration and invasion of pancreatic ductal adenocarcinoma. J Cell Mol Med. 2019; 23:5006–16. https://doi.org/10.1111/jcmm.14352. PMID:31119886
