Oncoscience
Bridging clinical insight and laboratory model in high-grade serous ovarian carcinoma (HGSOC) using DNA sequencing-based profiling of TP53
Faisal Iqbal1
1Department of Pharmaceutical Sciences, University of Illinois Chicago, Chicago, IL 60612, USA
Correspondence to: Faisal Iqbal, email: faisalsaleh59@ymail.com, faisali@uic.edu, https://orcid.org/0000-0001-7596-9180
Keywords: HGSOC; OVCAR3; TP53
Received: June 12, 2025
Accepted: October 06, 2025
Published: October 14, 2025
Copyright: © 2025 Iqbal. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
The most prevalent and aggressive subtype of epithelial ovarian cancer is high grade serous ovarian carcinoma (HGSOC) which is characterized by late-stage diagnosis and poor prognosis, and it accounts for approximately 70% of all ovarian cancer cases. The OVCAR3 cell line serves as a valuable in vitro model for studying the molecular mechanisms underlying the disease. In this study the Sanger sequencing method was used to detect DNA sequences, specifically the TP53 gene, making it ideal for comparing clinical and laboratory data. In this study, drug repurposing agents metformin, chlorpromazine (CPZ) alone, and a combination of the two, were tested on both clinical and laboratory ovarian cancer samples to evaluate hemocytometer and clonogenic assays for dead cells and proliferation, respectively. Following drug treatment, both samples were further analyzed using Sanger sequencing to detect TP53 profiling. The resulting data were analyzed to achieve successfully-known target regions and worked as a bridge between clinical and laboratory models. The insights gained from this study not only validate OVCAR3 as a representative model for HGSOC, but also provide a foundation for developing targeted therapeutic strategies.
INTRODUCTION
The most prevalent and aggressive subtype of epithelial ovarian cancer is high grade serous ovarian carcinoma (HGSOC) - approximately 70% of all ovarian cancer cases. HGSOC presents noteworthy challenges in treatment and prognosis, characterized by rapid development and late phase diagnosis. HGSOC is a complex molecular landscape involving a multitude of genetic alterations that drive tumorigenesis and therapeutic resistance [1–7].
The OVCAR3 cell line serves as a valuable in vitro model for studying the molecular mechanisms underlying the disease while retaining key genetic features of HGSOC, including TP53 and making it an ideal candidate for profiling studies. The Sanger sequencing method was used to enable the detection of specific DNA sequences, and the methodology offers high sensitivity and accuracy, making it suitable for identifying targeted genes [8–13].
The OVCAR3 clinical and laboratory samples were used to evaluate the data needed to implement the hemocytometer and clonogenic assay for drug repurposing, while Sanger sequencing was used for TP53 gene profiling and for comparing both clinical and laboratory data. In conclusion, Sanger sequencing provides a powerful approach for genetic landscaping of HGSOC that are linked to tumorigenesis and therapeutic resistance. This unified methodology holds promise for the advancement of targeted therapies and personalized medication in the fight against high grade serous ovarian carcinoma (HGSOC) [14–19].
RESULTS AND DISCUSSION
OVCAR3 cell line of TP53 gene
The study of high-grade serous ovarian carcinoma (HGSOC) OVCAR3 cell line is a widely utilized in vitro model in advanced-stage ovarian cancer. OVCAR3 retains key molecular characteristics, including the TP53 gene and makes it a precious tool for investigating the molecular mechanisms for HGSOC and assessing potential therapeutic approaches.
Comparing drug repurposing of clinical and laboratory sample data
The hemocytometer was performed first during cell suspension 24 hours before the use of the drug, the second time after cell culture media changed, and the third time 24 hours after using the drug to check the drug’s cytotoxicity in the OVCAR3 cell line. The final volume in each 6 WP (well plate) was 2 ml.
The hemocytometer cell count data presented in Table 1 provides insight into the responses of OVCAR3 cells to individual and combined drug treatments with metformin and CPZ.
Clinical sample drug repurposing data
In the first control group, the baseline dead cell count on day 1 was 2 × 104, which increased modestly to 3 × 104 after media change on day 2 and day 4 remained consistent 3 × 104 dead cell counting and indicating no drug interference. Metformin at 0.5 µM initially had 4 × 104 cells dropping to 2 × 104 after the media change, but then surging to 12 × 104 after 24 hours of drug exposure suggesting a delayed cell proliferation. Similarly, CPZ at 2 µM started at 4 × 104, remained stable on day 2, and dead cells increased to 14 × 104 on day 4 after 24 hours of drug treatment. Interestingly, the combination treatment (0.5 µM metformin + 2 µM CPZ) started with 3 × 104 dead cells, increased slightly to 4 × 104 on day 2, and then showed dead cells increase to 16 × 104 by day 4. Table 1 data indicates that at low concentrations both drugs alone and combined may have even triggered proliferation and only a few dead cell were counted.
Laboratory sample drug repurposing data
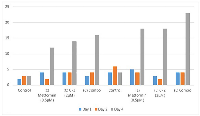
In the Table 1 laboratory control group, dead cells increased steadily from 4 × 104 on day 1, day 2 was 6 × 104 and day 4 dropped to 4 × 104. Metformin at 0.5 µM started at 5 × 104, remained stable (4 × 104) on day 2, and sharply increased dead cells to 18 × 104 on day 4, again indicating drug exposure. CPZ at 2 µM began at 3 × 10, dropped to 2 × 10 then 18 × 10. Notably, the combo treatment (0.5 µM metformin + 2 µM CPZ) had 4 × 10 cells on day 1 and day 2, which then rose to 23 × 10 on day 4, the highest dead cells observed in this group. These results suggest that drug repurposing did not suppress cell proliferation but might have triggered adaptive responses in OVCAR3 cells (Figure 1).
Clonogenic assay comparing clinical and laboratory sample data of OVCAR3 cell line
After drug treatment, the clonogenic assay was used for counting colonies to evaluate the data.
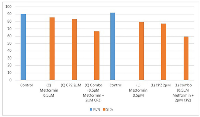
The clonogenic assay clinical sample results presented in Table 2, evaluate the cytotoxic effects of Metformin, CPZ and their combination on OVCAR3 cells. The first control yielded 90 colonies, representing a 90% plating efficiency (PE). Treatment with metformin at 0.5 µM (20 µl) reduced the colony count to 77, resulting in a surviving fraction (SF) of 85.55%, while CPZ at 2 µM (20 µl) produced 75 colonies with an SF of 83.33%. The combination of metformin and CPZ further reduced the colonies to 60, corresponding to a significantly lower SF of 66.66%.
In Table 2 laboratory sample, the control produced 92 colonies (PE = 92%), while metformin at 0.5 µM (20 µl) and CPZ at 2 µM (20 µl) reduced colony numbers to 73 and 71, resulting in SF values of 79.35% and 77.17% respectively. The combination treatment (0.5 µM metformin + 2 µM CPZ, 20 µl each) further decreased colony formation to 55 colonies, corresponding to SF of 59.78% (Figure 2).
Sanger sequencing-based profiling of TP53
In this study, Sanger sequencing was employed to characterize TP53 status in OVCAR3 cell line, a widely used in vitro model for HGSOC. The successful sequencing was performed to compare the wild type clinical sample with wild type laboratory sample. Sanger sequencing remains the gold standard method and is used for known-target sequences where high precision and base-calling quality are needed. The sequencing chromatogram of both wild type clinical and wild type laboratory samples were obtained from a reverse primer to confirm the accuracy of the data. The chromatograms base-calling quality was fast and clear with minimal noise in the background. The sequencing alignment reads the TP53 which well-characterized the HGSOC. The findings were consistent and validated the methodology. Sanger sequencing remains desirable for targeted low throughput analysis with high base precision. It is highly suitable when confirming the wild type target sequence with a limited number of samples. It is simple, cost effective and has less bioinformatics requirements and make it approachable for most molecular laboratories. So the Sanger sequencing application for TP53 profiling provided reliable and high quality data and confirmed the comparable wild type. These outputs not only validated the genetic profile of OVCAR3 cell line but is also helpful in ovarian cancer research.
Clinical implication
The OVCAR3 cell line identification provides opportunities for targeted therapeutic intrusions with comprehensive molecular profiling to guide personalized treatment approaches. This study engaged Sanger sequencing to investigate the OVCAR3 cell line landscape for high grade serous ovarian carcinoma (HGSOC). The Figure 3 findings align with existing literature and underscores the utility of OVCAR3 in bridging laboratory models with clinical insights. The Sanger sequencing analysis identified a TP53 gene in OVCAR3 cell line, a trademark of HGSOC. The use of Sanger sequencing allowed for fast and quantitative detection of nucleotides within a precise target region of TP53. This technique offers numerous advantages including higher sensitivity for detecting low-frequency variants. Particularly, Sanger sequencing is highly suitable for genetic detection and information that can be helpful for understanding the tumor.
The current study demonstrates the value of OVCAR3 as an appropriate in vitro model for TP53 oncogene and drug resistance in HGSOC. The integration of molecular approaches such as Sanger sequencing into preclinical workflows strengthens the relevance of cell-based assays and ensures that genetic context is correctly considered during drug screening. The recent study suggests that the efficacy of certain drug combinations including repurposing agents like chlorpromazine (CPZ) and metformin may vary significantly depending on TP53 status. In conclusion, Sanger sequencing based analysis confirmed the comparable clinical and laboratory wild type TP53 gene, this approach offers a fast and consistent technique for gene profiling and can be adapted for broader use in ovarian cancer research and diagnostics.
MATERIALS AND METHODS
OVCAR3 cell line
The OVCAR3 cell line (ATCC) was cultured in RPMI-1640 medium supplemented with fetal bovine serum (FBS) 10% and penicillin-streptomycin 1%. At 37°C cells were sustained with 5% CO2.
Hemocytometer
A manual cell-counting device call hemocytometer was commonly used to define cell feasibility especially dead cells. In this study culture OVCAR3 cells were harvested, trypsinization for adherent cells and resuspended for suitable volume of PBS or culture medium. A 1:1 ratio mixture of the cell suspension with 0.4% trypan blue dye was prepared to differentiate between live and dead cells. Live cells reject the dye and appear clear while dead cells take up the dye and appear blue. A cell-suspension stain was loaded 10 µl on hemocytometer and the grid defined the known volume. The chamber filled the cell suspension with capillary action then cells were counted under a microscope in a specific square. Only cells within the square and those touching the upper and left borders are counted to avoid duplication. The number of cells per ml was calculated by multiplying the average cell count per square by the dilution factor with a standard conversion factor (10). Evaluation of cell-feasibility was obtained by dividing the number of live cells by the total number of cells and multiplying by 100 to get a percentage. This protocol provides a reliable, low-cost method for quantifying both total and viable cells in a given population. The purpose of using hemocytometer was to count the dead cell and check the impact of drugs that creating resistance or cytotoxicity.
Clonogenic assay
A gold-standard method, clonogenic assays were used for the evaluation of OVCAR3 ovarian cancer cell line for proliferation and survival of cancer cells after exposure of drug treatment. In this study, OVCAR3 cells were first cultured under standard conditions in RPMI-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Once the cells reached exponential growth, they were trypsinized and counted using a hemocytometer with different time frames in different conditions and seeded at low densities in 6-well plates to ensure that colonies arose from single cells. After allowing the cells to adhere overnight, the media was changed and the next day, cells were treated with the desired drugs CPZ, metformin and combo to compare with control (no drug) for a defined duration (commonly 24–72 hours) according to the cells condition. During this period, 50 or more cells were considered per colony. At the end of the incubation period, the medium was gently removed and cells were fixed with methanol and stained with 0.5% crystal violet for 15 minutes. Distilled water was used to wash off excess stain, and plates were air-dried. Colonies were counted manually on fluorescent microscope. The plating efficiency (PE) and surviving fraction (SF) were calculated to evaluate the treatment of cytotoxic effects. PE is the percentage of seeded cells that form colonies in untreated controls while SF is the ratio of colonies formed after treatment to the number of cells seeded, normalized to PE. This assay provides a sensitive measure of a treatment’s ability to inhibit reproductive viability in cancer cells over time.
DNA extraction
OVCAR3 cell line of TP53 gene, and clinical laboratory sample DNA was extracted to perform the application of PCR and Sanger sequencing. The combo of both sample cell plates were used to extract the DNA. First culture the OVCAR3 cells in RPMI-1640 medium supplemented with fetal bovine 10% and 1% penicillin-streptomycin at 37°C for 5% CO2. When the cell confluence reached 80%, the medium was aspirated and the cells were washed with 1 ml of sterile PBS. Cells were detached using 0.25% trypsin-EDTA and neutralized with fresh medium. The suspension was transferred into a 15 ml centrifuge tube and spun at 1000 × g for 2 minutes to pellet the cells. The supernatant was discarded and the pellet was resuspended in 200 µl of PBS. DNA was then extracted using a silica column-based kit such as the Qiagen DNeasy Blood and Tissue Kit. The resuspended cell pellet was lysed by adding 20 µl of Proteinase K and 200 µl of Buffer AL, followed by incubation at 55°C for 10 minutes to ensure complete cell lysis. After lysis, 200 µl of 100% ethanol was added to precipitate DNA and the mixture was transferred to a spin column. The column was centrifuged and the bound DNA was washed with Buffer AW1 and AW2 to remove proteins and salts. Finally, DNA was eluted in 50 µl of AE buffer or nuclease-free water.
The purity and concentration of the DNA were evaluated on NanoDrop.
Primer design
Target genes commonly found in HGSOC clinical and laboratory samples, including TP53 were selected for gene profiling. Primers were synthesized by Integrated DNA Technologies (IDT, USA). Using forward primer (CCCATGGCATCCTAGTGAAA) and reverse primer (CAAAGGTCCGGAAGTTGTGG). Reverse primer was also used for Sanger sequencing.
PCR reaction setup for Sanger sequencing
Polymerase chain reaction (PCR) was performed to generate a DNA template for Sanger sequencing. The total reaction volume was 25 µl for each sample. The reaction mixture contained 10 ng of DNA template, dNTP mix (10 mM each) 0.5 µl, 10X PCR buffer 2.5 µl, 25 mM MgCl2 for 1.5 µl, 0.5 µl each of forward and reverse primers (10 µM), Taq DNA polymerase (5 U/µL) 0.25 µl and nuclease-free water to complete the final volume. Thermal cycling was carried out under the following conditions: the initial denaturation at 94°C for 3 minutes with followed by 30 amplification cycles of denaturation at 94°C for 30 seconds, primer annealing at 54°C for 30 seconds and extension at 72°C for 30 seconds with a final extension step at 72°C for 5 minutes. PCR products were then purified to remove residual primers, nucleotides, enzymes and buffer components using a silica column-based purification kit (QIAquick PCR Purification Kit, Qiagen). The purified amplicons were quantified and approximately 10 µl of PCR product (probably 10 ng/µl) was submitted for Sanger sequencing along with 5 µl of sequencing primer (5 µM), ensuring that only one primer was used per reaction.
Sanger sequencing of TP53 in OVCAR3 cells
The sequencing reactions were performed using an automated capillary electrophoresis system (e.g., Applied Biosystems 3500) and BigDye™ Terminator v3.1 Cycle Sequencing Kit. Raw sequencing chromatograms were analyzed on software.
CONCLUSIONS
In conclusion, these findings enhance our understanding of the cellular and molecular mechanisms driving HGSOC and inform the development of targeted therapies. The OVCAR3 model continues to be instrumental in bridging laboratory research and clinical applications, offering valuable insights into the complexities of ovarian cancer.
AUTHOR CONTRIBUTIONS
F.I: Conceptualization, methodology, investigation, visualization, writing original draft, resource, review and editing.
CONFLICTS OF INTEREST
The author has no conflicts of interest to declare.
FUNDING
No funding was used for this paper.
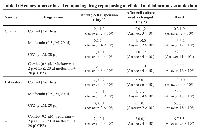
- 1. Novel ovarian cancer maintenance therapy targeted at mortalin and mutant p53. Int J Cancer. 2020; 147:1086–97. https://doi.org/10.1002/ijc.32830. PMID:31845320
- 2. Targeted DNA sequencing of high-grade serous ovarian carcinoma reveals association of TP53 mutations with platinum resistance when combined with gene expression. Int J Cancer. 2024; 155:104–16. https://doi.org/10.1002/ijc.34908. PMID:38447012
- 3. XTP8 Promotes Ovarian Cancer Progression by Activating AKT/AMPK/mTOR Pathway to Regulate EMT. Cell Biochem Biophys. 2024; 82:945–57. https://doi.org/10.1007/s12013-024-01246-4. PMID:38717641
- 4. Application of single cell sequencing technology in ovarian cancer research (review). Funct Integr Genomics. 2024; 24:144. https://doi.org/10.1007/s10142-024-01432-w. PMID:39196391
- 5. Single-cell transcriptomics reveals the aggressive landscape of high-grade serous carcinoma and therapeutic targets in tumor microenvironment. Cancer Lett. 2024; 593:216928. https://doi.org/10.1016/j.canlet.2024.216928. PMID:38714290
- 6. Aulosirazole Stimulates FOXO3a Nuclear Translocation to Regulate Apoptosis and Cell-Cycle Progression in High-Grade Serous Ovarian Cancer (HGSOC) Cells. Mol Pharmacol. 2024; 106:145–54. https://doi.org/10.1124/molpharm.124.000921. PMID:39079718
- 7. The CHK1 inhibitor prexasertib in BRCA wild-type platinum-resistant recurrent high-grade serous ovarian carcinoma: a phase 2 trial. Nat Commun. 2024; 15:2805. https://doi.org/10.1038/s41467-024-47215-6. PMID:38555285
- 8. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013; 4:2126. https://doi.org/10.1038/ncomms3126. PMID:23839242
- 9. Ovarian cancer cell line panel (OCCP): clinical importance of in vitro morphological subtypes. PLoS One. 2014; 9:e103988. https://doi.org/10.1371/journal.pone.0103988. PMID:25230021
- 10. Comprehensive genomic profiling of high-grade serous ovarian carcinoma from Chinese patients identifies co-occurring mutations in the Ras/Raf pathway with TP53. Cancer Med. 2019; 8:3928–35. https://doi.org/10.1002/cam4.2243. PMID:31124283
- 11. Genomic analysis of genetic heterogeneity and evolution in high-grade serous ovarian carcinoma. Oncogene. 2010; 29:4905–13. https://doi.org/10.1038/onc.2010.245. PMID:20581869
- 12. Characterisation of Ovarian Cancer Cell Line NIH-OVCAR3 and Implications of Genomic, Transcriptomic, Proteomic and Functional DNA Damage Response Biomarkers for Therapeutic Targeting. Cancers (Basel). 2020; 12:1939. https://doi.org/10.3390/cancers12071939. PMID:32709004
- 13. RPA using a multiplexed cartridge for low cost point of care diagnostics in the field. Anal Biochem. 2018; 547:84–88. https://doi.org/10.1016/j.ab.2018.02.010. PMID:29447855
- 14. RPA-Based colorimetric detection of SARS-Cov-2 (Covid-19) and its physiological effects. Int J Health Sci. 2023; 6. https://doi.org/10.53730/ijhs.v6nS7.13862.
- 15. Epidemiology and Resistance Pattern in Microbial Pneumonia: A Review: Epidemiology and Resistance Pattern in Microbial Pneumonia. Pak J Health Sci. 2022; 3:27–31. https://doi.org/10.54393/pjhs.v3i05.229.
- 16. Enhancing the effectiveness of Chimeric Antigen Receptor (CAR) T cells against tumors through CRISPR/Cas9-mediated PD-1 disruption. Int J Health Sci. 2023; 7:1836–50. https://doi.org/10.53730/ijhs.v7nS1.14397.
- 17. CRISPR/Cas9-based manipulation of oncogenic chromosomal changes in vivo and drug impact on blood pressure. Int J Health Sci. 2023; 7:2130–39. https://doi.org/10.53730/ijhs.v7nS1.14461.
- 18. Outcomes of Women With High-Grade and Low-Grade Advanced-Stage Serous Epithelial Ovarian Cancer. Obstet Gynecol. 2017; 129:439–47. https://doi.org/10.1097/AOG.0000000000001867. PMID:28178043
- 19. Identification of Novel Somatic TP53 Mutations in Patients with High-Grade Serous Ovarian Cancer (HGSOC) Using Next-Generation Sequencing (NGS). Int J Mol Sci. 2018; 19:1510. https://doi.org/10.3390/ijms19051510. PMID:29783665
