Oncoscience
When strokes reveal a hidden malignancy: An atypical case of metastatic colorectal cancer with extensive thromboembolism
Md Tanzim Ahsan1 and Sultana Azreen2
1Wrightington, Wigan and Leigh (WWL) Teaching Hospitals, NHS Foundation Trust, The United Kingdom
2Clatterbridge Cancer Centre, Clatterbridge Rd, Birkenhead, Wirral, Merseyside, The United Kingdom
Correspondence to: Md Tanzim Ahsan, email: md.ahsan@wwl.nhs.uk
Keywords: trousseau’s syndrome; cancer-associated stroke (CAS); hypercoagulability; colorectal cancer; systemic thromboembolism
Received: July 01, 2025
Accepted: September 05, 2025
Published: September 15, 2025
Copyright: © 2025 Ahsan and Azreen. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
Cancer-associated thromboembolism and Trousseau’s syndrome, which are paraneoplastic hypercoagulable states, are significant yet frequently underrecognized causes of cryptogenic stroke. We report a 71-year-old male who presented with recurrent embolic strokes and systemic thrombosis, later diagnosed with metastatic colorectal adenocarcinoma. Despite extensive investigations, no initial cancer-related symptoms were noted, and the diagnosis was made retrospectively after supraclavicular lymph node biopsy. The patient exhibited multi-territory infarcts, deep vein thrombosis, and renal and splenic infarcts, highlighting the aggressive thrombotic potential of malignancy-associated stroke. This case highlights the importance of early consideration of occult malignancy in cryptogenic embolic events, supporting comprehensive oncologic evaluation for unexplained hypercoagulable states.
INTRODUCTION
Cancer poses a considerable burden to global health, with an estimated lifetime risk of 40% and a reported approximately 20 million new cancer cases and 9.7 million cancer-related deaths worldwide in 2022 [1, 2]. Aside from its direct impact, malignancy is responsible for systemic sequelae, one of which is a heightened risk of thromboembolism, which presents as ischemic stroke [3]. Cancer-related thromboembolism, including Trousseau’s Syndrome and Cancer-Associated Stroke (CAS), is becoming increasingly recognized as a significant clinical concern. Research indicates that nearly 10% of ischemic stroke cases may be linked to an underlying cancer [4]. The risk of ischemic stroke is significantly elevated in cancer patients, particularly in the months preceding or following a cancer diagnosis, indicating a potential link between cryptogenic strokes and occult malignancy [5].
Cancer-associated thrombosis is caused by several mechanisms, such as hypercoagulability, non-bacterial thrombotic endocarditis (NBTE), direct invasion of the vessel, and endothelial dysfunction due to chemotherapy [4]. Among these, NBTE poses a particularly alarming risk for ischemic stroke as it leads to embolization without any signs of a bacterial infection [3]. In a large autopsy study, cerebrovascular disease was detected in 14.6% of cancer patients, with nearly half of these strokes being clinically silent [5]. Additionally, systemic cancer enhances thrombin generation, promoting both venous and arterial thrombosis [4].
Systemic thrombosis, like multifocal arterial and venous embolism, also hinders early diagnosis, postponing the diagnosis of an underlying malignancy [6]. This is also compounded by the fact that cancer screening approaches in stroke are contentious, and there is no standard approach to finding occult malignancies in stroke patients [5]. For instance, colorectal cancer, despite being among the most common malignancies worldwide, is less frequently implicated in stroke-related thromboembolism compared to lung or pancreatic cancers [1, 7]. However, a recent study of over 7.5 million cancer patients found colon cancer to be among the leading malignancies to cause fatal strokes [1].
We present a unique case of metastatic colon adenocarcinoma that manifested with recurrent embolic strokes and significant thrombosis. This highlights the necessity of maintaining a high suspicion for cancer in patients experiencing cryptogenic thromboembolic events. By sharing this case, we aim to enhance the existing literature on cancer-induced strokes and emphasize the necessity for improved screening guidelines for individuals presenting with cryptogenic strokes and systemic thrombosis.
CASE PRESENTATION
A 71-year-old male was admitted to the hospital on December 31, 2024, with a three-week history of generalized malaise, left-sided headache, and swelling of the left upper limb. Although symptoms raised initial suspicion for vasculitis or autoimmune etiologies due to limb swelling and headache, he did not report associated visual disturbances, nausea, vomiting, or neurological deficits. He denied experiencing pain while chewing or touching his face and did not have any symptoms suggestive of temporal arteritis. There were no night sweats, significant weight loss, or symptoms indicative of systemic infection, although he had been experiencing intermittent hot flushes. His only gastrointestinal symptom was occasional constipation, but he denied diarrhea, abdominal pain, or urinary complaints.
His past medical history was significant for hypertension, type 2 diabetes mellitus, ulcerative colitis, and osteoarthritis. His regular medications included atorvastatin 40 mg once daily, amlodipine 10 mg once daily, indapamide 2.5 mg once daily, sulfasalazine 500 mg twice daily, ramipril 10 mg once daily, and metformin 500 mg modified-release twice daily. He lived with his wife, was a non-smoker, and consumed alcohol occasionally. He had been independently mobile with the use of a walking stick but had noted a recent decline in mobility due to worsening knee pain.
On examination, his vital signs revealed a blood pressure of 175/75 mmHg, heart rate of 88 beats per minute, respiratory rate of 20 breaths per minute, and oxygen saturation of 97% on room air. He was afebrile at the time of presentation, with a recorded temperature of 37.6°C. He was alert and oriented, and there were no signs of acute distress. Cardiovascular examination was unremarkable, and auscultation of the chest revealed good bilateral air entry with no evidence of consolidation. Examination of the head and neck identified right submandibular lymph node enlargement, which was non-tender and non-matted, as well as a non-pulsatile, non-tender left supraclavicular swelling, raising suspicion for lymphadenopathy. There were no palpable axillary lymph nodes. A notable generalized swelling of the left upper limb was present, with preserved power and sensation. Additionally, a cystic swelling was observed over the left elbow. There were no oral ulcers or dental abscesses, and throat examination was unremarkable. On inspection of the chest, telangiectasia was noted over the left pectoral region. His abdominal examination was normal, and he exhibited a wobbly gait.
Investigations and initial workup
Initial blood work demonstrated a markedly elevated C-reactive protein (CRP) at 119 mg/L, along with leukocytosis (WBC 15 × 10⁹/L, with neutrophils 12.3 × 10⁹/L). Hemoglobin was 110 g/L, and platelets were within normal limits at 161 × 10⁹/L. His kidney function was preserved, with an estimated glomerular filtration rate (eGFR) above 90 mL/min/1.73 m², although his serum potassium was low at 2.8 mmol/L. His random blood glucose was elevated at 9.7 mmol/L.
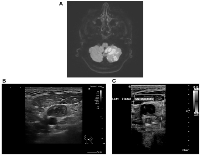
A chest X-ray showed no evidence of consolidation. Electrocardiography (ECG) revealed normal sinus rhythm, and a focused assessment with sonography for trauma (FAST) was negative. Given his neurological symptoms, an MRI of the brain was performed, which demonstrated multifocal acute infarctions in multiple vascular territories, most consistent with a cardioembolic event (Figure 1A).
Progression and further workup
Following admission, his clinical course raised concerns regarding an underlying prothrombotic state. A computed tomography (CT) scan of the neck, chest, abdomen, and pelvis performed on December 31, 2024, revealed multiple enlarged left supraclavicular and cervical lymph nodes, extensive intra-abdominal para-aortic lymphadenopathy, and several nodular lesions in the anterior pararenal space, raising suspicion for a malignant lymphoproliferative disorder (Figure 1B). In addition, extensive thrombosis was noted in the left internal jugular vein and subclavian vein. There was also evidence of wedge-shaped bilateral hypodense cortical areas in the middle and lower thirds of both kidneys, suggestive of renal infarcts.
Further Doppler ultrasonography on January 2, 2025, confirmed extensive deep vein thrombosis (DVT) in the left upper limb (Figure 1C). A repeat CT scan on January 9, 2025, showed significant progression of the mesenteric and para-aortic lymphadenopathy, reinforcing concerns for an underlying malignancy, particularly lymphoma. Additional findings included a newly identified splenic infarct and persistent renal infarcts. Pulmonary emboli were also noted, though of small volume.
Given the constellation of embolic events, the hematology team initiated therapeutic anticoagulation with Dalteparin. Cardiology was consulted, and an echocardiogram performed on December 30, 2024, ruled out infective endocarditis, showing no valvular vegetations. A serial troponin trend revealed an initial elevation (1354 ng/L), which subsequently declined (1206 ng/L to 974 ng/L), making an acute coronary syndrome less likely. Telemetry monitoring did not detect any episodes of atrial fibrillation.
The patient’s condition continued to deteriorate, prompting a biopsy of the left supraclavicular lymph node on January 17, 2025. Histopathological analysis revealed metastatic adenocarcinoma with immunohistochemical markers strongly positive for AE1/AE3, CK20, and CDX2, suggesting a colorectal primary. The malignant cells were negative for CK7, TTF-1, Napsin A, GATA 3, and PAX 8. Based on these findings, further investigation into a primary colorectal malignancy was recommended.
Hospital course and outcome
Despite medical management, the patient continued to decline, with progressive lymphadenopathy and worsening systemic symptoms. His clinical condition raised concerns about a new cerebrovascular event, prompting an urgent CT of the head on January 10, 2025, which confirmed an acute left parietal infarct, along with subacute infarctions in the left cerebellar hemisphere and occipital lobe. Given his multiple embolic episodes and high suspicion of an underlying malignancy, he remained on anticoagulation, which was transitioned from dalteparin to apixaban 5 mg twice daily at discharge (see Table 1 for an overview of clinical findings).
Following discussions with the stroke team, the hematology service, and his family, a decision was made to focus on palliative care. Given his rapid clinical deterioration and poor performance status, chemotherapy was considered but ultimately ruled out by multidisciplinary consensus, and he was subsequently discharged under a Fast Track discharge pathway to a nursing home. A multidisciplinary team (MDT) discussion on February 3, 2025, confirmed that he was not a candidate for further intervention due to his advanced disease. He remained under supportive care.
DISCUSSION
This case report describes a 71-year-old male who presented with recurrent embolic strokes and systemic thrombosis, ultimately diagnosed with metastatic colorectal adenocarcinoma. Despite lacking overt cancer symptoms, the patient experienced multiple embolic strokes and extensive systemic thrombosis, eventually diagnosed retrospectively with metastatic colorectal adenocarcinoma. This case highlights diagnostic challenges and emphasizes inflammation-driven malignancy transformation, underscoring the importance of early suspicion for occult malignancy in cryptogenic thromboembolism. The case illustrates the importance of maintaining a high index of suspicion for occult cancer in patients presenting with unexplained stroke and systemic thrombosis. Earlier suspicion or targeted screening could have potentially enabled timely initiation of oncologic therapy, possibly influencing clinical outcomes.
Trousseau’s syndrome (TS), a paraneoplastic hypercoagulability syndrome, is considered a subset of CAS, primarily manifesting as arterial and venous thromboembolism in cancer patients. The relationship between TS and CAS is critical, as both conditions share common coagulation pathway activations driven by tumor-secreted factors [8]. Kitamura et al. described TS in hematologic malignancies, where embolic strokes resulted from hypercoagulability linked to cytokine-driven endothelial activation [8]. Similarly, Chen et al. presented cases where systemic thrombosis preceded cancer diagnosis, reinforcing the importance of comprehensive oncologic screening in embolic stroke patients without clear etiologies [9] (Table 2).
The mechanisms underlying CAS are largely driven by cancer-induced hypercoagulability, with malignancies promoting systemic thrombosis through increased thrombin generation, platelet activation, and procoagulant microparticles [10]. Elevated tissue factor (TF) expression in tumor cells accelerates clot formation, while neutrophil extracellular traps (NETs) further contribute by providing a scaffold for thrombus development. Currently, the measurement of TF and soluble P-selectin remains largely within research settings rather than routine clinical practice [10]. Key differences in biomarker profiles between Trousseau’s syndrome and non-cancer-associated thromboembolism are summarized in Table 3. Studies have demonstrated significantly higher NET levels in CAS patients, correlating with D-dimer elevations, which serve as potential biomarkers for detecting occult malignancies. Future research and clinical trials targeting NETs may offer promising avenues for therapeutic intervention in CAS [11]. In some cases, nonbacterial thrombotic endocarditis (NBTE) leads to sterile vegetations on cardiac valves, which embolize and cause multi-territory infarcts, as seen in our patient [12]. While tumor embolism is rare, it has been reported in colorectal cancer, further complicating stroke etiology [3, 10].
Certain imaging and laboratory results differentiate CAS from other embolic strokes. Atrial fibrillation-free multi-territory infarcts are very suggestive of a hypercoagulable state [3]. Additionally, D-dimer levels are always elevated in CAS compared to the typical stroke mechanisms [11]. Management is contentious, with DOACs having proven to be of limited benefit in recurrent CAS prevention, and LMWH remaining the anticoagulant of choice for malignancy-related thrombosis [12]. With advanced illness in our patient, treatment prioritized supportive care over intense stroke prophylaxis.
The cases reported by Chen et al. and Meng et al. further demonstrated that cancer-associated embolic strokes often present with multiple infarcts in various vascular territories, reinforcing the importance of hypercoagulability assessment in cryptogenic strokes [10, 13]. Additionally, Tasi et al. and Liu et al. reported cases of gastrointestinal malignancies leading to refractory thromboembolism, similar to our patient’s presentation [14, 15]. These studies highlight the aggressive thrombotic potential of mucin-producing tumors and the need for early identification to guide therapeutic decisions (Table 2).
The importance of multimodal treatment strategies is underscored by Wakabayashi et al., who found that patients receiving concurrent cancer treatment and anticoagulation had significantly better outcomes than those managed with anticoagulation alone [16]. This suggests that treating the underlying malignancy is as crucial as anticoagulation in controlling CAS progression. Additionally, Tasi et al. highlighted the limited efficacy of standard anticoagulation in some cases, suggesting that adjunctive therapies targeting tumor-induced coagulation pathways may be beneficial [14] (Table 2).
Future research needs to maximize risk stratification using biomarkers like NETs and identify optimal anticoagulation therapy to improve CAS outcomes. Further investigations into the efficacy of alternative anticoagulants, such as novel direct thrombin inhibitors, may provide better long-term management strategies. Additionally, prospective studies assessing the utility of advanced imaging techniques, such as PET-CT and thrombus histopathology, could refine early cancer detection in patients presenting with embolic strokes of unknown origin. Ultimately, a multidisciplinary approach integrating neurology, oncology, and hematology expertise is essential to improving diagnostic accuracy and treatment efficacy for patients with CAS and TS.
CONCLUSIONS
This case highlights the challenges of diagnosing Trousseau’s syndrome and the connection between idiopathic thromboembolism and malignancy, as well as the need for early screening for cancer in patients presenting with cryptogenic strokes. The patient’s recurring embolic events, deep vein thrombosis, and systemic infarctions were ultimately diagnostic of metastatic colorectal cancer, illustrating the challenge of diagnosing Trousseau’s syndrome. Although anticoagulation remains the cornerstone of therapy, recurrences are common, and more effective screening and treatment strategies are needed. Future research should be directed at the optimization of diagnostic approaches and anticoagulation regimes. Multidisciplinary approach is the cornerstone of the improvement of results in cancer-related stroke and hypercoagulability patients.
AUTHOR CONTRIBUTIONS
MTA and SA collected the data, including medical images and clinical information, and wrote the original draft. MTA advised on patient treatment. MTA analyzed patient data. All authors read and approved the final version of the manuscript.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
CONFLICTS OF INTEREST
Authors have no conflicts of interest to declare.
ETHICAL STATEMENT
The present study was conducted in accordance with the guidelines of The Declaration of Helsinki to ensure that patient information was not misused, and privacy information was not leaked, in order to protect the rights and interests of the patient. Written informed consent was obtained from the patient during admission.
CONSENT
Written informed consent was obtained from the patient for publication of the data and the images in this case report during the admission. The consent to publish the case report was also obtained from the immediate relatives due to the deceased patient.
FUNDING
No funding was used for this paper.


- 1. Stroke among cancer patients. Nat Commun. 2019; 10:5172. https://doi.org/10.1038/s41467-019-13120-6. PMID:31729378
- 2. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024; 74:229–63. https://doi.org/10.3322/caac.21834. PMID:38572751
- 3. Cancer-associated stroke: Pathophysiology, detection and management (Review). Int J Oncol. 2019; 54:779–96. https://doi.org/10.3892/ijo.2019.4669. PMID:30628661
- 4. Cancer-Related Stroke: An Emerging Subtype of Ischemic Stroke with Unique Pathomechanisms. J Stroke. 2020; 22:1–10. https://doi.org/10.5853/jos.2019.02278. PMID:32027788
- 5. Thrombogenesis-associated genetic determinants as predictors of thromboembolism and prognosis in cervical cancer. Sci Rep. 2023; 13:9519. https://doi.org/10.1038/s41598-023-36161-w. PMID:37308506
- 6. A case report of Trousseau syndrome. Medicine (Baltimore). 2023; 102:e34449. https://doi.org/10.1097/MD.0000000000034449. PMID:37505132
- 7. Trousseau’s Syndrome: A Paraneoplastic Complication. Cureus. 2024; 16:e66969. https://doi.org/10.7759/cureus.66969. PMID:39156994
- 8. Trousseau’s syndrome in diffuse large B-cell lymphoma. EJHaem. 2023; 5:271–73. https://doi.org/10.1002/jha2.837. PMID:38406545
- 9. Trousseau syndrome-induced cerebral infarction: Two case reports. Medicine (Baltimore). 2024; 103:e40937. https://doi.org/10.1097/MD.0000000000040937. PMID:39686462
- 10. Cancer-related stroke: Exploring personalized therapy strategies. Brain Behav. 2022; 12:e2738. https://doi.org/10.1002/brb3.2738. PMID:35938982
- 11. Cancer-associated ischemic stroke: current knowledge and future directions. Bleeding Thromb Vasc Biol. 2024; 3. https://doi.org/10.4081/btvb.2024.117.
- 12. Mechanisms of Ischemic Stroke in Patients with Cancer: A Prospective Study. Ann Neurol. 2021; 90:159–69. https://doi.org/10.1002/ana.26129. PMID:34029423
- 13. Trousseau syndrome with recurrent cerebral infarction as the first oneset in a gastrointestinal malignant tumor patient: A case report. Medicine (Baltimore). 2024; 103:e40146. https://doi.org/10.1097/MD.0000000000040146. PMID:39470525
- 14. Trousseau’s syndrome related to adenocarcinoma of the colon and cholangiocarcinoma. Eur J Neurol. 2004; 11:493–96. https://doi.org/10.1111/j.1468-1331.2004.00814.x. PMID:15257690
- 15. A case of Trousseau syndrome: Screening, detection and complication. Open Life Sci. 2024; 19:20220824. https://doi.org/10.1515/biol-2022-0824. PMID:38465339
- 16. Prognosis of pancreatic cancer with Trousseau syndrome: a systematic review of case reports in Japanese literature. J Egypt Natl Canc Inst. 2023; 35:40. https://doi.org/10.1186/s43046-023-00202-2. PMID:38093170
- 17. Changes in Serial D-Dimer Levels Predict the Prognoses of Trousseau’s Syndrome Patients. Front Neurol. 2018; 9:528. https://doi.org/10.3389/fneur.2018.00528. PMID:30018592
- 18. Tissue factor: a neglected role in cancer biology. J Thromb Thrombolysis. 2022; 54:97–108. https://doi.org/10.1007/s11239-022-02662-0. PMID:35763169
- 19. Tissue factor and cell signalling in cancer progression and thrombosis. J Thromb Haemost. 2011 (Suppl 1); 306–15. https://doi.org/10.1111/j.1538-7836.2011.04318.x. PMID:21781267
- 20. Pathophysiology of Trousseau’s syndrome. Hamostaseologie. 2015; 35:52–59. https://doi.org/10.5482/HAMO-14-08-0037. PMID:25403091
- 21. The role of P-selectin in cancer-associated thrombosis and beyond. Thromb Res. 2022 (Suppl 1); S22–28. https://doi.org/10.1016/j.thromres.2021.12.027. PMID:36210556
- 22. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Blood. 2008; 112:2703–8. https://doi.org/10.1182/blood-2008-02-142422. PMID:18539899
- 23. Thrombin generation, thrombin-antithrombin complex, and prothrombin fragment F1+2 as biomarkers for hypercoagulability in cancer patients. Thromb Res. 2020; 186:80–85. https://doi.org/10.1016/j.thromres.2019.12.018. PMID:31918352
- 24. Elevated levels of prothrombin fragment 1 + 2 indicate high risk of thrombosis. Clin Appl Thromb Hemost. 2008; 14:279–85. https://doi.org/10.1177/1076029607309176. PMID:18160575
- 25. Much More than Trousseau Syndrome. The Broad Spectrum of the Pancreatic Paraneoplastic Syndromes. Pathol Oncol Res. 2018; 24:1–10. https://doi.org/10.1007/s12253-017-0206-6. PMID:28160197
- 26. Prothrombin fragment 1+2 and thrombin-antithrombin complex levels in patients with inherited APC resistance due to factor V Leiden mutation. Br J Haematol. 1996; 92:435–41. https://doi.org/10.1046/j.1365-2141.1996.d01-1500.x. PMID:8603014
- 27. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015; 29:17–24. https://doi.org/10.1016/j.blre.2014.09.003. PMID:25294122
- 28. Cancer-related coagulopathy (Trousseau’s syndrome): review of the literature and experience of a single center of internal medicine. Clin Exp Med. 2013; 13:85–97. https://doi.org/10.1007/s10238-013-0230-0. PMID:23456539
